PITIRIASIS ROSADA, BUSCANDO EL AGENTE CAUSAL
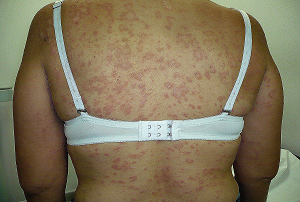
La Pitiriasis rosada fue descrita en el año 1860 por el dermatólogo Francés Camile Gibert, en el año 1860; enfermedad eritematosa y descamativa, exantematosa, que afecta niños adolescentes y adultos, mas frecuente en el sexo femenino.
En la mayoría de las actualizaciones y búsquedas que hagas por la internet encontraras que la enfermedad esta ocasionada por los virus Herpes 6 y 7, con una duración que puede durar de 8 a 10 semanas, 3 meses e incluso mas.
Pero yo te traigo acá unos estudios donde investigadores encontraron otros agentes causales de la misma, por lo tanto todavía algunos científicos consideran que su origen viral sigue estando en duda.
Estos agentes son:
1.) Legionella Micdadei, (pneumophila), bacteria Gram (-) involucrada en la llamada Neumonía de Pittsburg, Enfermedad del Legionario y también en la llamada Fiebre de Pontiac.
2.) virus del tipo Picornavirus (involucrados en el refriado común).
3.) Virus de Epstein Barr. (EBV), también perteneciente a la familia de los herpes virus y agente causal de la mononucleosis infecciosa.
4.) Micoplasmas, (bacteria-Mycoplasma Pneumoniae) también descritos en asociación con pitiriasis rosada
Con estos ejemplos descritos se cae la afirmación de que la Pitiriasis Rosada de Gibert sea ocasionada únicamente por los ya mencionados herpes virus 6 y 7.
Aquí encuentras la actualización sobre la LA PITIRIASIS ROSADA, ACTUALIZACION (2025)
Saludos,,,
Dr. José Lapenta.
Dr. José M. Lapenta.
ENGLISH
Pityriasis rosea was described in 1860 by the dermatologist Frances Camile Gibert, in the year 1860; erythematous and scaly, exanthematous disease, which affects children, adolescents and adults, more frequent in the female sex.
In most of the updates and searches you do on the internet you will find that the disease is caused by the Herpes 6 and 7 viruses, with a duration that can last from 8 to 10 weeks, 3 months or even more.
But I bring you here some studies where researchers found other causal agents of the same, therefore some scientists still consider that its viral origin is still in doubt.
These agents are:
1.) Legionella Micdadei, (pneumophila), Gram (-) bacteria involved in the so-called Pittsburgh Pneumonia, Legionnaire's Disease and also in the so-called Pontiac Fever.
2.) Picornavirus type viruses (involved in the common cold).
3.) Epstein Barr virus (EBV), also belonging to the herpes virus family and the causative agent of infectious mononucleosis.
4.) Mycoplasmas, (bacteria-Mycoplasma Pneumoniae) also described in association with pityriasis rosea
With these examples described, the claim that Gibert's Pityriasis Rosea is caused only by the aforementioned herpes viruses 6 and 7 falls apart.
Here you will find the update on the THE PITYRIASIS ROSEA UPDATE (2025)
Dr. Jose Lapenta R.
Dr. José M. Lapenta.
EDITORIAL ESPANOL:
====================
Hola amigos DERMAGICOS, de nuevo con ustedes. LA PITIRIASIS ROSADA, enfermedad ancestral descrita por GIBERT hace muchos años, todavía no se le conoce causa especifica. En los últimos años se han publicado algunos trabajos tratando de buscarle un agente causal, pero todavía no hay una asociación agente-enfermedad comprobada 100%. Estas referencias nos muestran algunos de esos trabajos.
El la lista RX-DERM, hace unas semanas se publico un paciente tratado con VALTREX (valaciclovir), con excelentes resultados. Asociación con herpes virus ???, o bacilos ???, el tiempo y futuras investigaciones terminaran de aclarar el panorama.
Saludos,,,
Dr. Jose Lapenta R.,,,
EDITORIAL ENGLISH:
===================
Hello DERMAGICS friends, again with you. THE PITYRIASIS ROSEA, ancestral illness described many years ago by GIBERT, is not still known the cause. In the last years some works have been published trying to look for a causal agent, but there still is not an association agent-illness proven 100%. These references show us some of those works.
The list RX-DERM, has published a patient treaty with VALTREX(valaciclovir) some weeks ago, with excellent results. Association with herpesvirus ???, or bacilluses ???, the time and future investigations will clarify the query.
Greetings,,,
Dr. José Lapenta R.
************************************
************************************
======================================================================
DERMAGIC/EXPRESS(33)
======================================================================
PITIRIASIS ROSADA, BUSCANDO UN AGENTE CAUSAL
PITYRIASIS ROSEA, LOOKING FOR A CAUSAL AGENT
=======================================================================
1.) Pityriasis rosea Gibert: detection of Legionella micdadei antibodies in
patients.
2.) Legionella species.
3.) Absence of picornavirus genome in pityriasis rosea.
4.) Human herpesvirus 7 in pityriasis rosea [letter]
5.) Pityriasis rosea and human herpesvirus 7, a true association? [letter]
6.) Human herpesvirus 7 in patients with pityriasis rosea. Electron microscopy investigations and polymerase chain reaction in mononuclear cells, plasma and skin.
7.) Human herpesvirus 7: antigenic properties and prevalence in children and
adults.
8.) Human herpesvirus types 6, 7, and 8.
9.) [Histopathologic, ultrastructural, immunologic and virologic study of
Gibert's pityriasis rosea]
10.) Description of Epstein-Barr Virus.
=========================================================================
1.) Pityriasis rosea Gibert: detection of Legionella micdadei antibodies in
patients.
=========================================================================
AU: Gjenero-Margan-I; Vidovic-R; Drazenovic-V
AD: Epidemiology Service, Croatian National Institute of Public Health,
Zagreb, Croatia.
SO: Eur-J-Epidemiol. 1995 Aug; 11(4): 459-62
ISSN: 0392-2990
PY: 1995
LA: ENGLISH
CP: NETHERLANDS
AB: Some epidemiological and clinical characteristics of Pityriasis rosea
Gibert has led us to hypothesize that this disease may be the clinical
manifestation of an infection caused by legionellas. We have thus tested
the sera of 36 patients ill with Pityriasis rosea and 19 controls for
Legionella pneumophila serogroup 1-6 and Legionella micdadei antibodies.
These, who had the same age and sex distribution as study patients, were
receiving treatment for other diseases in the same ward. Also tested were
200 sera from the voluntary blood donors from the same region as study
patients. Legionella micdadei antibodies were detected in 12 (33.3%)
Pityriasis rosea cases and in one (5.2%) control. They were significantly
more common in Pityriasis rosea cases than in either controls or voluntary
blood donor population. The findings to date encourage continued research
into the causative relationship between the Legionella micddadei infection
and the onset of Pityriasis rosea Gibert.
=========================================================================
2.) Legionella species
=========================================================================
Source: Mandell, Douglas and Bennett's
Principles and Practice of Infectious Diseases Fourth Edition.
Legionellae are gram-negative aerobic bacilli that share a number of common
phenotypic features, including growth on buffered charcoal yeast extract
(BCYE) agar, lack of growth on blood agar, catalase activity, and
requirement for cysteine. Tests for urease, nitrate reduction, and
fermentative activity are uniformly negative. 7 Although individual species
differ in several phenotypic characteristics, such as gelatin
liquefication, hippurate hydrolysis, and oxidase activity, these tests are
of limited use in differentiation. When grown on yeast extract agar,
Legionella spp. produce a water-soluble, extracellular compound that
fluoresces yellow-green on exposure to long-wave ultraviolet light. Several
species exhibit a blue-white or red autofluorescence under ultraviolet
light. Most species produce b-lactamase; L. micdadei, L. maceachaernii, and
L. feeleii do not. Cell wall fatty acid profiles and ubiquinone content are
sufficiently distinctive to permit species identification on the basis of
gas-liquid chromatography. 8,8a Differentiation of the common species is
most conveniently made in the laboratory by direct fluorescent antibody
staining of the isolates. Slide agglutination can also be used for selected
isolates. 1,9 Determination of DNA homology is the definitive method
especially for the less common strains.
Legionella micdadei is unique in that it retains the modified acid-fast
stain. 2,3 Legionella micdadei can appear as weakly or partially acid-fast
bacilli in clinical specimens. The acid-fast property is not usually
present in organisms grown on solid media, but may be retained in liquid
culture. The modified acid-fast stain substitutes 1% sulfuric acid (a less
potent decolorizing agent) for the traditional 3% hydrochloric acid in
alcohol. This characteristic has occasionally led to misidentification of
L. micdadei infection as mycobacterial infection, with initiation of
antituberculous agents. 3,10,11
Based on differences in DNA sequence homology and DNA guanine and cytosine
content, Garrity and Brown proposed division of the family into three
genera, Legionella (L. pneumophila), Fluoribacter (F. bozemanae, F.
gormanii, F. dumoffii), and Tatlockia (T. micdadei, T. maceachaernii).
12,13 However, commonly accepted usage includes only the single genus,
Legionella. 14,14a
========================================================================
3.) Absence of picornavirus genome in pityriasis rosea.
========================================================================
Aractingi S; Morinet F; Mokni M; Tieng V; Flageul B; Fermand JP; Dubertret L
Unite de Dermatologie, Hopital Tenon, Paris, France.
Arch Dermatol Res (GERMANY) Dec 1996 289 (1) p60-1 ISSN: 0340-3696
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9706
Subfile: INDEX MEDICUS
Tags: Human
=========================================================================
4.) Human herpesvirus 7 in pityriasis rosea [letter]
=========================================================================
Drago F; Ranieri E; Malaguti F; Losi E; Rebora A
Lancet (ENGLAND) May 10 1997 349 (9062) p1367-8 ISSN: 0140-6736
Language: ENGLISH
Document Type: LETTER
Journal Announcement: 9708
Subfile: AIM; INDEX MEDICUS
Tags: Human
=========================================================================
5.) Pityriasis rosea and human herpesvirus 7, a true association? [letter]
=========================================================================
Author
Lebb´e C; Agbalika F
Source
Dermatology, 196(2):275 1998
=========================================================================
6.) Human herpesvirus 7 in patients with pityriasis rosea. Electron microscopy investigations and polymerase chain reaction in mononuclear cells, plasma and skin.
=========================================================================
Author
Drago F; Ranieri E; Malaguti F; Battifoglio ML; Losi E; Rebora A
Address
Institute of Dermatology, University of Genoa, Italy.
Source
Dermatology, 195(4):374-8 1997
Abstract
BACKGROUND: Clinical evidence suggests a viral etiology for pityriasis
rosea (PR). OBJECTIVE: To evaluate human herpesvirus (HHV)-6 and HHV-7 as
candidates for the etiology of PR. METHODS: Blood and skin tissue from 12
patients with acute PR, and 12 patients with other dermatoses were studied,
as well as blood samples from 25 healthy persons. Serum interferon
(IFN)-alpha and IFN-gamma were analyzed by ELISA. Analysis of morphological
changes in cocultured peripheral blood mononuclear cells (PBMC) and
electron microscopy (EM) to identify viral particles were performed.
Polymerase chain reaction (PCR) with specific primers for HHV-6 and HHV-7
DNA sequences was performed on the plasma and PBMC of patients and healthy
controls and on the skin of patients with PR and other skin diseases.
RESULTS: PR plasma contained detectable IFN-alpha and IFN-gamma, whereas
plasma from controls did not. PBMC from PR patients showed ballooning cells
and syncytia after 7 days in culture whereas PBMC from controls and
recovered PR patients did not. This cytopathic effect was also documented
in a PR patient who relapsed and in Sup-T1 cell cultures inoculated with
the cell-free supernatant from centrifuged cultured PBMC; in this
supernatant, herpesvirus, virions were detected by EM, PCR identified HHV-7
DNA in PBMC, plasma and skin from all patients with active PR and in the
PBMC only of 5 patients tested 10-14 months later. Weaker signals of HHV-7
DNA were detected in PBMC of 11 controls, but not in their plasma. Skin was
negative for HHV-7 in all control specimens. CONCLUSIONS: Although the
detection of HHV-7 DNA in PBMC and tissues does not prove directly a causal
role, HHV-7 DNA in cell-free plasma corresponds to active replication which
supports a causal relationship. We propose that PR is a clinical
presentation of HHV-7 reactivation.
========================================================================
7.) Human herpesvirus 7: antigenic properties and prevalence in children and
adults.
========================================================================
Author(s) Wyatt LS; Rodriguez WJ; Balachandran N; Frenkel N
Address Laboratory of Viral Diseases, National Institute of Allergy and
Infectious Diseases, Bethesda, Maryland 20892.
Source J Virol 1991;65:6260 - 5.
Abstract The recent isolation of human herpesvirus 7 (HHV-7) from activated
CD4+ T lymphocytes of a healthy individual raises questions regarding the
prevalence of this virus in humans and its immunological relationship to
previously characterized human herpesviruses. We report that HHV-7 is a
ubiquitous virus which is immunologically distinct from the highly
prevalent T-lymphotropic HHV-6. Thus, (i) only two of six monoclonal
antibodies to HHV-6 cross-reacted with HHV-7-infected cells, (ii) Western
immunoblot analyses of viral proteins revealed different patterns for
HHV-6- and HHV-7-infected cells, (iii) tests of sequential serum samples
from children revealed seroconversion to HHV-6 without concomitant
seroconversion to HHV-7, and (iv) in some instances HHV-7 infection
occurred in the presence of high titers of HHV-6 antibodies, suggesting the
lack of apparent protection of children seropositive for HHV-6 against
subsequent infection with HHV-7. On the basis of the analyses of sera from
children and adults it can be concluded that HHV-7 is a prevalent human
herpesvirus which, like other human herpesviruses, infects during
childhood. The age of infection appears to be somewhat later than the very
early age documented for HHV-6.
========================================================================
8.) Human herpesvirus types 6, 7, and 8
========================================================================
Source: Harrison's 14 1.998
Human herpesvirus (HHV) type 6 was first isolated in 1986 from peripheral-blood leukocytes of six persons with various lymphoproliferative disorders. Although initially thought to be B-lymphotropic and thus designated human B-lymphotropic virus, HHV-6 is clearly primarily T-lymphotropic. The virus has a worldwide distribution, and two genetically distinct variants (HHV-6A and HHV-6B) are now recognized.
Infection with HHV-6 frequently develops during infancy as maternal antibody wanes. Although HHV-6A has not yet been associated with disease, HHV-6B can cause exanthem subitum (roseola infantum), a common illness characterized by fever with subsequent rash. HHV-6B is also a major cause of febrile seizures without rash during infancy. In older age groups, HHV-6B has been associated with mononucleosis syndromes, focal encephalitis, and (in immunocompromised hosts) pneumonitis and disseminated disease. As many as 80 percent of adults are seropositive for HHV-6. The virus may be transmitted by saliva and possibly by genital secretions. There is no established treatment or vaccine.
HHV-7 was isolated in 1990 from T lymphocytes from the peripheral blood of a healthy 26-year-old man. Other isolates have since been obtained. It appears that the virus is frequently acquired during childhood and is frequently present in the saliva of healthy adults. No human disease has yet been definitively linked to HHV-7, although some cases of exanthem subitum have been associated with HHV-7 infection.
Unique herpesvirus-like DNA sequences were reported during 1994 and 1995 in tissues derived from Kaposi's sarcoma and body cavity-based lymphoma occurring in patients with AIDS. These sequences are partially homologous to the DNA of Epstein-Barr virus and herpesvirus saimiri of squirrel monkeys. When subjected to representational-difference analyses, more than 90 percent of Kaposi's sarcoma tissue samples were found to contain these sequences, whereas appropriate control tissues did not. The same herpesvirus-like DNA sequences have been reported in Kaposi's sarcoma tissue from non-AIDS patients, in a subgroup of AIDS-related B-cell body cavity-based lymphomas, in certain brain tumors, and in some proliferative skin lesions of organ transplant recipients. Approximately 15 percent of non-Kaposi's-sarcoma tissue specimens from patients with AIDS contain these sequences, which have also been found in semen from both AIDS and non-AIDS patients. Because of the uniqueness of these sequences, some authors have tentatively called the virus from which they come HHV-8. Its role in Kaposi's sarcoma and other diseases remains to be established. The recent isolation of HHV-8 in cell culture should help define its role in disease by making diagnostic techniques more reliable.
========================================================================
9.) [Histopathologic, ultrastructural, immunologic and virologic study of
Gibert's pityriasis rosea]
========================================================================
Author
Bonaf´e JL; Icart J; Perp`ere M; Oksman F; Divoux D
Source
Ann Dermatol Venereol, 109(10):855-61 1982
Abstract
A complete study of pityriasis rosea (Gibert) has been performed; this
study includes: --a pathological and ultrastructural examination of the
skin biopsies; --an immunological study: direct and indirect
immunofluorescence (seric anti-cutaneous antibodies); --a virological
investigation: ultrastructural examination of the specimens, inoculation of
homogenized-skin specimens to 3 cellular cultures; --serological
investigations for influenza A, B, parainfluenza 1, 2, 3, adenovirus,
respiratory syncitial virus, Mycoplasma pneumoniae, ornithosis-psittacosis,
Q-fever, herpes-virus, herpes-virus varicellae, cytomegalovirus,
Epstein-Barr virus. All viral investigations had a negative result. Virus
or virus-like particle has were not detected on the ultrastructural
examinations. A viral infective agent cannot be incriminated as the cause
of PRG. However, a large number of PRG patients had antibodies for the
Epstein-Barr virus early antigen. The authors are carrying on with this
particular investigation.
======================================================================
10.) Description of Epstein-Barr Virus
======================================================================
Source: Mandell, Douglas and Bennett's
Principles and Practice of Infectious Diseases Fourth Edition.
Physical Properties
-------------------
Epstein-Barr virus has the characteristic morphology of the Herpesviridae family of viruses of which it is a member. By electron microscopy, individual virions are 180–200 nm in diameter and appear as hexagonal nucleocapsids surrounded by a complex envelope. The nucleocapsids are 100 nm in diameter and consist of an orderly array of capsomeres with the 5:3:2 symmetry seen in herpesviruses. 20 EBV DNA is double stranded with a molecular weight of 101 ± 3 X 106 and a buoyant density of 1.718 g/cm in CsCl. 21 The EBV genome encodes about 80 proteins. 22
Biologic Properties
-------------------
The host range of the virus is quite limited. In vitro cultivation of the virus has been described only in B lymphocytes and nasopharyngeal epithelial cells of humans and certain nonhuman primates. 23 The virus generally does not produce cytopathic effects in infected cells. After infection by the virus, B lymphocytes that contain the EBV genome are capable of continuous in vitro cultivation, and are termed transformed or immortalized. EBV has been confirmed as the transforming agent by the detection of viral antigens by indirect immunofluorescence within the nuclei of transformed cells or by hybridization of cellular DNA with purified EBV DNA.
Epstein-Barr virus receptors are demonstrable on B lymphocytes and nasopharyngeal epithelial cells of humans and certain nonhuman primates. 24,25 EBV receptors are also present on a smaller proportion of complement receptor–bearing, non-B, non-T lymphocytes. 26-28 The EBV receptor has recently been identified as the receptor for the d region of the third component of complement. 29,30 The C3d receptor (also known as CR2 or CD21), a 145-kD glycoprotein, is encoded by a member of a multiple gene family that specifies a number of cell membrane molecules.
After attachment to the receptor, the virus gains entry to susceptible B lymphocytes. Before the detection of virus-directed protein synthesis, Epstein-Barr nuclear antigens (EBNA) are demonstrable in nuclei of infected cells. 31 At high multiplicities of infection, up to 25 percent of EBV-exposed B lymphocytes express EBNA. 32 Viral DNA synthesis is initiated with the production of multiple copies of the EBV genome. In transformed cells from patients with infectious mononucleosis or Burkitt's lymphoma, some viral DNA may be incorporated into the DNA of the host cell, although most of the DNA remains in a circular nonintegrated form as an episome. Linear integration of EBV DNA into host cell DNA may be enhanced by stimulation of the host cells by B-cell mitogens such as bacterial lipopolysaccharide at the time of transformation. 33 The host cell gains the property of immortality whether the virus is present in integrated, in episomal, or in a combination of the two forms. 33 Immortalization of B lymphocytes is a complex process that involves a coordinated interplay among a number of viral and host cell gene products. 22,34-42 Latent infection of B cells by EBV is determined by binding of the EBNA-1 protein to a viral promoter termed oriP. EBNA-1 also transactivates the EBNA-2 protein, which, in turn, activates production of two EBV-encoded latent membrane proteins (LMP-1 and -2), as well as several B-cell gene products (CD21, CD23 and c-fgr). LMP-1 activates production of several intercellular adhesion cell proteins (ICAM-1, LFA-1, and LFA-3), as well as an autocrine growth factor for B cells (CD23). LMP-1 may play an important role in EBV-associated oncogenesis in that it morphologically transforms epithelial and B cells, 43 and prevents apoptosis (programmed cell death). 44
Under most culture conditions, 10 percent or fewer of EBV-exposed B lymphocytes form continuous cell lines. 32 After transformation, the host cell replicates, and the progeny cells contain several EBV genocopies in latent form. In addition to viral antigens, EBV-transformed B lymphocytes produce and/or secrete immunoglobulin. 45-48 Although most polyclonally transformed lines produce immunoglobulin of the IgM class, studies of clonally transformed B lymphocytes indicate that EBV is capable of induction of the synthesis of immunoglobulin of the IgG, IgA, or IgM classes. 49
Cell lines that contain EBV genomes are characterized as producer or nonproducer lines. Most of the time, EBV remains in latent form both in vitro in established cell lines and in vivo in circulating lymphocytes. Latent virus can be activated by stimulation of host B cells by certain chemicals, or by antibodies to surface immunoglobulin. 22 Following such stimulation, an immediate early gene of EBV (the EBV BZLF1 gene product or ZEBRA protein) is activated. Expression of this protein leads to a cascade of events culminating in the production of early EBV gene (EA) products responsible for viral replication (thymidine kinase and DNA polymerase), and late (structural) genes of the virus including viral capsid antigens (VCA). Upon induction of early and viral capsid antigens, the virus enters a lytic cycle of infection that results in both the production of progeny virions, and destruction of the host cell.
Epidemiology
Serum Antibody Prevalence
-------------------------
Antibodies to EBV have been found in all population groups studied, and most studies have shown no predilection for either sex. Antibodies are acquired earlier in life in tropical than in industrialized countries, but by adulthood 90–95 percent of most populations have demonstrable EBV antibodies. 50,51
Two strains of EBV have been defined on the basis of viral gene sequences expressed during latency, and on their ability to transform B lymphocytes. 22 The strains (termed Type 1 [A] or 2 [B]) are not distinguishable serologically, but they do express unique epitopes that are identified by cytotoxic T cells. Although it was initially thought that there were specific geographic distributions for these two strains of EBV, it is now clear that both strains are widely distributed, and that individuals can be co-infected with both strains. In the United States and in Great Britain, EBV seroconversion occurs before the age of 5 in about 50 percent of the population. 51-53 A second wave of seroconversion occurs midway through the second decade of life. EBV seroconversion may occur at a younger average age in the southern United States than in other areas of the country. 54 Lower socioeconomic groups have a higher EBV antibody prevalence than do more affluent age-matched controls. The reported increased prevalence of EBV antibodies among blacks probably reflects this socioeconomic distribution.
=====================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS No (33) 29/01/99 DR. JOSE LAPENTA R.
UPDATED 06/12/2025
=====================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela 1.998-2.025
Producido por Dr. José Lapenta R. Dermatólogo
Venezuela
1.998-2.025
Tlf: 0414-2976087 - 04127766810
04243431100


















.png)