LA TALIDOMIDA, EL REGRESO

ESPAÑOL:
Hola amigos DERMAGICOS, en la edición de hoy La TALIDOMIDA, un medicamento que en los años 50 causó una generación de niños con malformaciónes, había sido lanzado al mercado como antidepresivo el cual podía medicarse en mujeres embarazadas, LO QUE PROVOCÓ una OLA de niños con MALFORMACIONES CONGÉNITAS. Este producto, caído en desgracia desde esa época, fue retirado del mercado entre 1961 y 1963 en TODO EL MUNDO.
Con el tiempo se le fue demostrando su utilidad en variadas patologías dermatológicas, entre ellas la LEPRA, y otras no dermatológicas, y hoy día, el 16 de Julio de 1.998 fue aprobado por la FDA Americana, para el tratamiento de la REACCIÓN LEPROSA, y otras condiciones. En ESTAS REFERENCIAS bibliográficas, queda plasmado un poco la historia de este producto de la casa CELGENE.
ENGLISH:
Hello DERMAGIC friends, in today's edition THALIDOMIDE, a drug that in the 50s caused a generation of children with malformations, had been launched on the market as an antidepressant which could be used on pregnant women, WHICH CAUSED a WAVE of children with CONGENITAL MALFORMATIONS. This product, which has fallen into disgrace since that time, was withdrawn from the market between 1961 and 1963 throughout the WORLD.
Over time, its usefulness in various dermatological pathologies was demonstrated, including LEPROSY, and other non-dermatological ones, and today, on July 16, 1998, it was approved by the American FDA, for the treatment of LEPROSY REACTION, and other conditions. In THESE bibliographical REFERENCES, a little of the history of this product from the CELGENE company is reflected.
***********************************
****** DATA-MÉDICOS *********
*********************************
LA TALIDOMIDA, EL REGRESO, THE THALIDOMIDE'S COME BACK
**************************************
***** DERMAGIC-EXPRESS No 9 *****
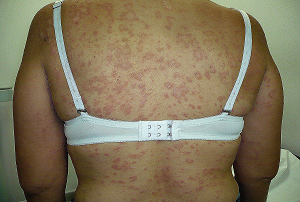
****** 23 OCTUBRE DE 1.998 *******
************************************
************************************
====================================================================
DERMAGIC/EXPRESS(9)
=====================================================================
LA TALIDOMIDA, EL REGRESO / THE THALIDOMIDE'S COME BACK
=====================================================================
1.) The thalidomide tragedy in Germany: the end of a historic
medicolegal trial.
2.) Thalidomide's long shadow [editorial]
3.) Damages awarded for thalidomide children.
4.) Normal child after maternal thalidomide ingestion in critical
period of pregnancy.
5.) Another chance for thalidomide?
6.) Crossover study of thalidomide vs placebo in Jessner's
lymphocytic infiltration of the skin.
7.) Treatment of cutaneous and pulmonary sarcoidosis with thalidomide.
8.) Thalidomide as treatment of refractory aphthous ulceration
related to human immunodeficiency virus infection.
9.) Amniotic band sequence in child of thalidomide victim [letter;
comment]
10.) Chronic cutaneous lupus erythematosus treated with thalidomide.
11.) Remission of Langerhans cell histiocytosis with thalidomide
treatment [letter]
12.) Thalidomide for the treatment of chronic graft-versus-host
disease [see comments]
13.) Thalidomide's effectiveness in erythema nodosum leprosum is
associated with a decrease in CD4+ cells in the peripheral blood.
14.) Lupus erythematosus profundus with partial C4 deficiency
responding to thalidomide.
15.) Pyoderma gangrenosum associated with Behcet's disease: treatment with thalidomide.
16.) Thalidomide in type-2 lepra reaction--a clinical experience.
17.) Combination thalidomide and cyclosporine for cardiac allograft rejection. Comparison with combination methylprednisolone and cyclosporine.
18.) Treatment of refractory rheumatoid arthritis--the thalidomide
experience.
19.) Successful treatment with thalidomide of acute graft-versus-host
20.) Thalidomide. A surprising recovery.
21.) Thalidomide: a novel therapy for microsporidiosis.
22.) A prospective trial of thalidomide for the treatment of
HIV-associated idiopathic esophageal ulcers.
23.) Thalidomide in the treatment of the cutaneous manifestations of lupus erythematosus: experience in sixteen consecutive patients.
24.) The effect of thalidomide on experimental tumors and metastases.
25.) Paradoxical effect of thalidomide prophylaxis on chronic graft-vs.-hostdisease.
26.) Thalidomide and recurrent aphthous stomatitis: a follow-up study.
27.) THALIDOMIDE, the product.
28.) FDA Clears Thalidomide For Leprosy
==================================================================
1.) TI - The thalidomide tragedy in Germany: the end of a historic
medicolegal trial.
SO - N Engl J Med 1971 Mar 4;284(9):481-2
AU - Curran WJ
===================================================================
===================================================================
2.) TI - Thalidomide's long shadow [editorial]
SO - Br Med J 1976 Nov 13;2(6045):1155-6
MJ - Drug Industry; Drug Therapy [adverse effects]
===================================================================
===================================================================
3.)TI - Damages awarded for thalidomide children.
SO - Lancet 1969 Aug 9;2(615):331-2
AU - Whicher H
MJ - Abnormalities, Drug-Induced [etiology]; Jurisprudence; Thalidomide
[adverse effects]
===================================================================
===================================================================
4.) TI - Normal child after maternal thalidomide ingestion in critical
period of pregnancy.
SO - Lancet 1970 Feb 7;1(641):275-7
AU - Pembrey ME; Clarke CA; Frais MM
MJ - Thalidomide [adverse effects]
MN - Child; Gestational Age; Pregnancy
===================================================================
===================================================================
5.) TI - Another chance for thalidomide?
SO - Lancet 1966 Apr 30;1(444):981-2
AU - Vladutiu A
MJ - Encephalomyelitis, Allergic [immunology]; Encephalomyelitis;
Immunosuppressive Agents [therapeutic use]; Thalidomide [therapeutic use]
===================================================================
===================================================================
6.) TI - Crossover study of thalidomide vs placebo in Jessner's
lymphocytic infiltration of the skin.
===================================================================
SO - Arch Dermatol 1995 Sep;131(9):1032-5
AU - Guillaume JC; Moulin G; Dieng MT; Poli F; Morel P; Souteyrand P;
Bonnetblanc JM; Claudy A; Daniel F; Vaillant L; et al
AD - Dermatology Service, Hopital Henri Mondor, Creteil, France.
MJ - Skin Diseases [drug therapy]; Thalidomide [therapeutic use]
MN - Adult; Cross-Over Studies; Double-Blind Method; Middle Age;
Prospective Studies; Thalidomide [adverse effects]
MT - Female; Human; Male; Support, Non-U.S. Gov't
PT - CLINICAL TRIAL; JOURNAL ARTICLE; MULTICENTER STUDY; RANDOMIZED
CONTROLLED TRIAL
AB - BACKGROUND AND DESIGN: An effective therapy is still unavailable for
Jessner-Kanof lymphocytic infiltration of the skin. Thalidomide's efficacy
was suggested in an open study. Twenty-eight patients were randomly
assigned to receive thalidomide (100 mg/d) or placebo over a period of 2
months and were then switched to the other treatment. RESULTS: After the
first period, 11 of 13 patients treated with thalidomide were in complete
remission (CR), and there were two failures. There was no CR in the
patients who received placebo (chi y2 = 17.5; P .0001). After the second
period, nine of 14 patients who had received thalidomide were in CR. Eleven
of the 13 patients who had received thalidomide during the first period
were given placebo (two were unavailable for follow-up). Ten of them were
in CR: four were still free of lesions at the end of the second period, and
six experienced a relapse of their lesions after a mean duration of 26 +/-
10 (SD) days. A total of 25 patients participated in the two study periods;
CR was observed in 19 (76%) after thalidomide therapy and in four (16%)
after treatment with placebo (chi y2 = 11.1; P .001). Of 27 patients who
received thalidomide, 16 (59%) were in CR after 1 month and 20 (74%) were
in CR after 2 months. Two patients treated with thalidomide experienced
neurologic changes that were not consistent with typical
thalidomide-induced neuropathy. CONCLUSIONS: A therapeutic regimen of
thalidomide administered at a dosage of 100 mg/d for 2 months is able to
suppress the clinical symptoms of Jessner-Kanof lymphocytic infiltration of
the skin. The long-term risk-benefit has still to be evaluated.
===================================================================
7.) TI - Treatment of cutaneous and pulmonary sarcoidosis with thalidomide.
===================================================================
SO - J Am Acad Dermatol 1995 May;32(5 Pt 2):866-9
AU - Carlesimo M; Giustini S; Rossi A; Bonaccorsi P; Calvieri S
AD - Department of Dermatology, University of Rome La Sapienza, Italy.
MJ - Sarcoidosis, Pulmonary [drug therapy]; Sarcoidosis [drug therapy];
Skin Diseases [drug therapy]; Thalidomide [therapeutic use]
MN - Aged; Middle Age; Sarcoidosis, Pulmonary [complications]; Sarcoma,
Kaposi's [complications]; Skin Diseases [complications]; Skin Neoplasms
[complications]
MT - Case Report; Female; Human
PT - JOURNAL ARTICLE
AB - Many therapeutic agents have been proposed for treatment of
steroid-resistant sarcoidosis. Because administration of low doses of
thalidomide has been successful in treating other inflammatory diseases, it
was used in a patient with systemic sarcoidosis who was unresponsive to
corticosteroids and in a patient with pulmonary sarcoidosis, in whom
Kaposi's sarcoma developed after a course of corticosteroid therapy.
Thalidomide, 200 mg/day for 2 weeks followed by 100 mg/day for 11 weeks,
was given. This treatment was effective in both patients. No adverse
reactions were observed. Thalidomide, 100 mg on alternate days, is still
being administered. No relapse has occurred. Thalidomide, particularly
because of its inhibition of the macrophage function, may be a useful
alternative therapy in steroid-resistant cases. In addition, the
correlation between the angiotensin-converting enzyme level and the
clinical improvement observed in our patients suggests a direct parallel
between angiotensin-converting enzyme and the activity of the granulomatous
process.
===================================================================
8.) TI - Thalidomide as treatment of refractory aphthous ulceration
related to human immunodeficiency virus infection.
===================================================================
SO - Clin Infect Dis 1995 Feb;20(2):250-4
AU - Paterson DL; Georghiou PR; Allworth AM; Kemp RJ
AD - Infectious Diseases Unit, Royal Brisbane Hospital, Herston,
Queensland, Australia.
MJ - HIV Infections [complications]; HIV-1; Stomatitis, Aphthous [drug
therapy]; Thalidomide [therapeutic use]
MN - Adult; Middle Age; Recurrence; Retrospective Studies; Stomatitis,
Aphthous [etiology]; Thalidomide [adverse effects]
MT - Human; Male
PT - CLINICAL TRIAL; JOURNAL ARTICLE
AB - In recent years, thalidomide has been used for the treatment of a
variety of ulcerative and immunologic conditions. Several previous reports
have suggested that thalidomide therapy is beneficial for patients with
aphthous ulceration related to human immunodeficiency virus (HIV)
infection. We describe the use of thalidomide in 20 HIV-infected patients
with oropharyngeal, esophageal, and rectal ulceration. Nineteen patients
had a dramatic response to thalidomide therapy, with both subjective and
objective abatement in the signs and symptoms of their ulcerative disease.
The standard treatment course was 200 mg of thalidomide for 14 days (the
drug was administered at night). Four patients required additional courses
of treatment because symptoms recurred after thalidomide therapy was
stopped. Side effects due to thalidomide included rash (5 patients),
peripheral neuropathy (1 patient), and excessive fatigue (1 patient). There
did not appear to be any adverse immunologic effects in thalidomide-treated
patients. The mechanism of the effect of thalidomide is uncertain, although
recent studies have suggested that thalidomide selectively inhibits the
production of tumor necrosis factor alpha.
===================================================================
9.) TI - Amniotic band sequence in child of thalidomide victim [letter;
comment]
CM - Comment on: BMJ 1994 Jun 18; 308(6944):1635-6; Comment on: BMJ 1994
Aug 13; 309(6952):477
SO - BMJ 1994 Nov 26;309(6966):1442
AU - Tenconi R; Clementi M; Notari L; Lo Vasco VR
MJ - Amniotic Band Syndrome [etiology]; Extremities [abnormalities]; Hand
Deformities, Congenital [etiology]; Prenatal Exposure Delayed Effects;
Thalidomide [adverse effects]
===================================================================
10.) TI - Chronic cutaneous lupus erythematosus treated with thalidomide.
SO - Arch Dermatol 1993 Dec;129(12):1548-50
AU - Holm AL; Bowers KE; McMeekin TO; Gaspari AA
AD - University of Rochester (NY) Medical Center.
MJ - Lupus Erythematosus, Discoid [drug therapy]; Scalp Dermatoses [drug
therapy]; Thalidomide [therapeutic use]
MN - Adult; Alopecia [drug therapy]; Prednisone [administration & dosage]
[therapeutic use]; Thalidomide [administration & dosage]
MT - Case Report; Female; Human
===================================================================
11.) TI - Remission of Langerhans cell histiocytosis with thalidomide
treatment [letter]
SO - Clin Exp Dermatol 1993 Sep;18(5):487
AU - Misery L; Larbre B; Lyonnet S; Faure M; Thivolet J
MJ - Histiocytosis, Langerhans-Cell [drug therapy]; Thalidomide
[therapeutic use]
==================================================================
12.) TI - Thalidomide for the treatment of chronic graft-versus-host
disease [see comments]
===================================================================
CM - Comment in: N Engl J Med 1992 Sep 3; 327(10):735
SO - N Engl J Med 1992 Apr 16;326(16):1055-8
AU - Vogelsang GB; Farmer ER; Hess AD; Altamonte V; Beschorner WE; Jabs
DA; Corio RL; Levin LS; Colvin OM; Wingard JR; et al
AD - Department of Oncology, Johns Hopkins University School of Medicine,
Baltimore, Md.
MJ - Graft vs Host Disease [drug therapy]; Immunosuppressive Agents
[therapeutic use]; Thalidomide [therapeutic use]
MN - Adolescence; Adult; Bone Marrow Transplantation [adverse effects];
Child; Chronic Disease; Graft vs Host Disease [mortality];
Immunosuppressive Agents [administration & dosage] [adverse effects];
Infection [complications]; Middle Age; Thalidomide [administration &
dosage] [adverse effects]
MT - Human; Support, Non-U.S. Gov't; Support, U.S. Gov't, P.H.S.
PT - CLINICAL TRIAL; JOURNAL ARTICLE
AB - BACKGROUND. Allogeneic bone marrow transplantation is an accepted
therapy for hematologic cancer, aplastic anemia, and inherited
immunodeficiencies. Chronic graft-versus-host disease (GVHD) is the
principal complication in patients surviving more than 100 days.
Thalidomide has been shown experimentally to be effective in treating GVHD.
METHODS. We treated 23 patients with chronic GVHD refractory to
conventional treatment and 21 patients with "high-risk" chronic GVHD
(identified as having at least two of the following three risk factors:
chronic GVHD that has evolved from acute GVHD, lichenoid skin or
mucous-membrane changes, and hepatic dysfunction. Such patients have a high
mortality rate.) with thalidomide in a dose that produced a plasma level of
5 micrograms per milliliter two hours after administration. Therapy was
continued for three months after a complete response or for six months
after a partial response. RESULTS. The overall actuarial survival of all
enrolled patients was 64 percent. Survival was 76 percent among the
patients receiving salvage therapy for refractory GVHD and 48 percent among
those with high-risk GVHD. A complete response was observed in 14 patients,
a partial response in 12 patients, and no response in 18. Side effects were
minor, most notably sedation in almost all patients. CONCLUSIONS. In this
preliminary trial, thalidomide appeared to be safe and effective for the
treatment of chronic GVHD. A trial comparing thalidomide with prednisone in
patients with newly diagnosed chronic GVHD will be required to demonstrate
its relative efficacy.
===================================================================
13.) TI - Thalidomide's effectiveness in erythema nodosum leprosum is
associated with a decrease in CD4+ cells in the peripheral blood.
===================================================================
SO - Lepr Rev 1992 Mar;63(1):5-11
AU - Shannon EJ; Ejigu M; Haile-Mariam HS; Berhan TY; Tasesse G
AD - Pharmacology Research Department, G.W. Long Hansen's Disease Center,
Carville, La 70721.
MJ - CD4-CD8 Ratio; Erythema Nodosum [drug therapy]; Leprosy, Lepromatous
[drug therapy]; Thalidomide [therapeutic use]
MN - Adult; Erythema Nodosum [immunology]; Leprosy, Lepromatous [immunology]
MT - Human; Male; Support, Non-U.S. Gov't
PT - JOURNAL ARTICLE
AB - Thalidomide is well documented as being an effective drug in the
treatment of erythema nodosum leprosum (ENL). The mechanism of action of
thalidomide in ENL as well as the pathogenesis of ENL are yet to be fully
determined. Lepromatous leprosy patients experiencing ENL have been
reported to have an increase in the ratio of CD4+ to CD8+ cells in their
blood and ENL skin lesions. Thalidomide has been shown to cause a decrease
in the ratio of CD4+ to CD8+ lymphocytes in the blood of healthy males.
This decrease was due to a significant reduction in the numbers of Cd4+
lymphocytes and an apparent increase in the numbers of CD8+ lymphocytes. In
this study, thalidomide's effectiveness in halting chronic ENL and
arresting a relapse into ENL was consistently associated with a decrease in
the numbers of CD4+ lymphocytes in the blood of 2 male lepromatous leprosy
patients.
===================================================================
14.) TI - Lupus erythematosus profundus with partial C4 deficiency
responding to thalidomide.
===================================================================
SO - Br J Dermatol 1991 Jul;125(1):62-7
AU - Burrows NP; Walport MJ; Hammond AH; Davey N; Jones RR
AD - St. John's Dermatology Centre, St. Thomas' Hospital, London, U.K.
MJ - Complement 4 [deficiency]; Panniculitis, Lupus Erythematosus [genetics]
MN - Adolescence; Complement 4a [deficiency]; Complement 4b [deficiency];
Panniculitis, Lupus Erythematosus [drug therapy] [pathology]; Pedigree;
Skin [pathology]; Thalidomide [therapeutic use]
MT - Case Report; Female; Human; Support, Non-U.S. Gov't
PT - JOURNAL ARTICLE
AB - A female patient with disfiguring lupus erythematosus profundus (LEP)
from the age of 13 years was found to have an isolated partial C4
deficiency, with reduced levels of both allotypes, C4A and C4B. A genetic
basis for the hypocomplementaemia was confirmed by a family study of
complement and HLA types which revealed heterozygous null alleles for C4A
and C4B in the proband. Marked improvement in her cutaneous lesions
occurred with thalidomide.
===================================================================
15.) TI - Pyoderma gangrenosum associated with Behcet's disease: treatment
with thalidomide.
SO - J Am Acad Dermatol 1990 Nov;23(5 Pt 1):941-4
AU - Rustin MH; Gilkes JJ; Robinson TW
AD - Department of Dermatology, Royal Free Hospital, London, England.
MJ - Behcet's Syndrome [complications]; Leg Ulcer [pathology]; Pyoderma
[complications]; Thalidomide [therapeutic use]
MN - Adult; Gangrene; Leg Ulcer [drug therapy]; Prednisolone
[administration & dosage] [therapeutic use]; Pyoderma [drug therapy]
[pathology]; Recurrence
===================================================================
16.)TI - Thalidomide in type-2 lepra reaction--a clinical experience.
===================================================================
SO - Indian J Lepr 1990 Jul-Sep;62(3):316-20
AU - Jadhav VH; Patki AH; Mehta JM
AD - Dr. Bandorawala Leprosy Hospital, Pune.
MJ - Leprosy, Lepromatous [drug therapy]; Thalidomide [therapeutic use]
MN - Adolescence; Adult; Anti-Inflammatory Agents, Non-Steroidal
[therapeutic use]; Drug Therapy, Combination; Middle Age; Thalidomide
[adverse effects]
MT - Human; Male
PT - CLINICAL TRIAL; JOURNAL ARTICLE
AB - A clinical experience of using thalidomide in type-2 lepra reaction
(ENL) in 90 male patients--57 with lepromatous leprosy (LL) and 33 with
borderline lepromatous leprosy (BL)--is described. All the patients
responded well although some took a longer time to improve. No major side
effects were observed except for giddiness in 10 and gastrointestinal
upsets in 7 patients. Thalidomide thus appears to be a very effective drug
in the treatment of severe type-2 lepra reaction and apart from its
historically well-documented embryopathic effects, does not seem to have
any other serious side effects in the patients under study.
===================================================================
17.) TI - Combination thalidomide and cyclosporine for cardiac allograft
rejection. Comparison with combination methylprednisolone and cyclosporine.
===================================================================
SO - Transplantation 1990 Jan;49(1):20-5
AU - Tamura F; Vogelsang GB; Reitz BA; Baumgartner WA; Herskowitz A
AD - Department of Cardiovascular Surgery, Johns Hopkins Medical
Institutions, Baltimore, Maryland 21205.
MJ - Cyclosporins [administration & dosage]; Graft Rejection [drug
effects]; Heart Transplantation; Methylprednisolone [administration &
dosage]; Thalidomide [administration & dosage]
MN - Drug Therapy, Combination; Immunosuppression; Rats, Inbred Lew; Rats;
Transplantation, Homologous
MT - Animal; Comparative Study; Male; Support, Non-U.S. Gov't; Support,
U.S. Gov't, P.H.S.
PT - JOURNAL ARTICLE
AB - Combination CsA with corticosteroids is the most commonly used
maintenance immunosuppressive regimen after cardiac transplantation,
although their high-toxicity profiles frequently limit their clinical
benefit. Immunosuppressive agents that would act synergistically with CsA
but without the toxicity profile of corticosteroids would be clinically
useful. Thalidomide was removed from the market due to its teratogenic
effects, although it has known immunomodulatory activity. The purpose of
this study was (1) to determine whether maintenance immunosuppression with
thalidomide and subtherapeutic doses of CsA can help prevent rat cardiac
allograft rejection; and (2) to compare its synergism with CsA to the
commonly used corticosteroid, methylprednisolone. ACI-LEW allografts were
all treated with subtherapeutic doses of CsA (10 mg/g/day, s.c.) for 4
days. When CsA was then discontinued, severe rejection developed by
posttransplant day 14. Group 1 received CsA alone. Group 2 received in
addition oral thalidomide 100 mg/day for 14 days. Groups 3, 4, and 5
received CsA and methylprednisolone (low dose: 0.2 mg/kg/day s.c.; moderate
dose: 2.0 mg/kg/day s.c.; and high dose: 20 mg/kg/day s.c. Twelve
histologic parameters of rejection were semiquantitatively graded 0-4, and
total pathology scores were determined. The combination of thalidomide and
subtherapeutic CsA significantly reduced the severity of myocardial
necrosis, interstitial inflammation, interstitial edema, and the total
pathology score. Thalidomide was found to be equally as effective as low-,
moderate-, and high-dose methylprednisolone. The results of this study
suggest the potential clinical role of CsA and thalidomide in maintenance
immunosuppressive regimens, thereby avoiding the use of corticosteroids.
===================================================================
18.) TI - Treatment of refractory rheumatoid arthritis--the thalidomide
experience.
===================================================================
SO - J Rheumatol 1989 Feb;16(2):158-63
AU - Gutierrez-Rodriguez O; Starusta-Bacal P; Gutierrez-Montes O
AD - Department of Medicine, Facultad de Salud, Universidad del Valle,
Hospital Universitario del Valle, Evaristo Garcia, Cali, Colombia.
MJ - Arthritis, Rheumatoid [drug therapy]; Thalidomide [therapeutic use]
MN - Adult; Aged; Edema [chemically induced]; Middle Age; Pain; Sleep
Stages [drug effects]; Thalidomide [adverse effects]
MT - Female; Human; Male
PT - JOURNAL ARTICLE
AB - In an open study, 17 patients (16 women, 1 man) with refractory or
severe rheumatoid arthritis were treated with thalidomide. Two withdrew
from the study in the first weeks. Thirteen patients received 531 +/- 63
mg/day of thalidomide for 18.8 +/- 8.8 weeks; in 2 the dose was 300 mg/day
during 62 and 65 weeks. Seven patients attained complete remission, 5
partial remission, and the last 3 no improvement at all. Remissions lasted
6 years in 1 patient, 2 years in 3, 1 year in one, and varied between 8
months and 8 weeks in 7. After relapse, 5 patients received a 2nd course of
treatment and attained remission again. This lasted 24, 10, and 9 months in
3; two are taking 100 mg/day of thalidomide as a maintenance dose and
remain asymptomatic after 36 and 30 months. The side effects were
drowsiness, constipation, hard swelling of the lower limbs, erythema of the
face and limbs with local pruritus or burning sensation, hair loss, cough,
nasal obstruction, fever, and skin and mucosal dryness. In 8 patients there
was mild eosinophilia (less than 10%) and in 2 leukopenia. A 33-year-old
woman showed amenorrhea up to 2 months after stopping treatment. After a
2nd course of treatment, 2 patients developed peripheral sensory
neuropathy, which resolved spontaneously in 6 months. We believe these
findings justify controlled trials with this agent.
===================================================================
19.) TI - Successful treatment with thalidomide of acute graft-versus-host
disease after bone-marrow transplantation [letter]
SO - Lancet 1988 Jan 16;1(8577):117
AU - Lim SH; McWhannell A; Vora AJ; Boughton BJ
MJ - Bone Marrow Transplantation; Bone Marrow [transplantation]; Graft vs
Host Disease [drug therapy]; Thalidomide [therapeutic use]
===================================================================
20.) Thalidomide. A surprising recovery.
===================================================================
Stirling D; Sherman M; Strauss S
Celgene Corporation, Warren, N.J., USA.
J Am Pharm Assoc (Wash) (UNITED STATES) May-Jun 1997 NS37 (3) p306-13
ISSN:
1086-5802
Language: ENGLISH
Document Type: JOURNAL ARTICLE; REVIEW; REVIEW, TUTORIAL
Journal Announcement: 9710
Subfile: INDEX MEDICUS
The epidemic of birth defects in Europe in the early 1960s attributed to
thalidomide led to stringent and unprecedented drug safety requirements in
many
countries. No definitive mechanism of action has been determined for the
biological
activities associated with thalidomide. Food and Drug Administration and
congressional concerns about the handling of clinical investigations of
thalidomide
led to sweeping new regulations for clinical trials. Thalidomide is
currently being
used clinically to treat such conditions as cachexia associated with HIV
and cancer,
mycobacterial disease, and autoimmune diseases. (38 References)
===================================================================
21.) Thalidomide: a novel therapy for microsporidiosis.
===================================================================
Sharpstone D; Rowbottom A; Francis N; Tovey G; Ellis D; Barrett M;
Gazzard B
Department of HIV/GUM, Chelsea and Westminster Hospital, London, England.
Gastroenterology (UNITED STATES) Jun 1997 112 (6) p1823-9 ISSN:
0016-5085
Language: ENGLISH
Document Type: CLINICAL TRIAL; JOURNAL ARTICLE
Journal Announcement: 9709
Subfile: AIM; INDEX MEDICUS
BACKGROUND & AIMS: Microsporidiosis is a common cause of chronic diarrhea
in human
immunodeficiency virus (HIV)-seropositive individuals and often does not
respond to
treatment. Fecal tumor necrosis factor alpha (TNF-alpha) is elevated in
microsporidiosis; therefore, thalidomide, an anti-TNF-alpha agent, was used
as
therapy. METHODS: Eighteen subjects with chronic diarrhea caused by
Enterocytozoon
bieneusi that had not responded symptomatically to albendazole and 1
untreated
subject with Encephalitozoon intestinalis received 1 month of thalidomide,
100 mg
nocte. Clinical response was assessed by stool frequency and body weight,
histological response by light microscopy with villus height/crypt depth
ratios and
electron microscopy, and immunologic response by fecal TNF-alpha level.
RESULTS:
Seven subjects with chronic diarrhea due to E. bieneusi had a complete
clinical
response, and 3 had a partial response to thalidomide. There was a
significant
decrease in stool frequency from 5.3 to 3.1 per day (P = 0.001), and weight
increased
significantly by 1.2 kg (P < 0.02). Thalidomide significantly increased
the villus
height/crypt depth ratio (1.95 to 2.07; P = 0.045) and number of abnormal
forms of
microsporidia (P < 0.01). Fecal TNF-alpha level nonsignificantly decreased
from 17.9
to 8.9 U/mL. There was apparent disruption of all stages of the life cycle
of E.
intestinalis. CONCLUSIONS: Thalidomide may be an effective therapy for
diarrhea and
weight loss from E. bieneusi.
===================================================================
22.) A prospective trial of thalidomide for the treatment of
HIV-associated idiopathic esophageal ulcers.
===================================================================
Alexander LN; Wilcox CM
Department of Medicine, Emory University School of Medicine, Atlanta,
Georgia, USA.
AIDS Res Hum Retroviruses (UNITED STATES) Mar 1 1997 13 (4) p301-4
ISSN: 0889-
2229
Language: ENGLISH
Document Type: CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED
TRIAL
Journal Announcement: 9709
Subfile: INDEX MEDICUS
Thalidomide appears to be highly effective for oropharyngeal apthous
ulcers in HIV-
infected patients. However, there are limited data regarding the use of
this drug
for the treatment of HIV-associated idiopathic esophageal ulcer(s) (IEU).
Twelve HIV-
infected patients with esophageal symptoms and IEU as defined by previously
proposed
criteria were studied prospectively. Two of these patients had failed oral
corticosteroid treatment, and two others had a previous history of IEU.
Patients
were treated with thalidomide (200 mg/day orally) for 28 days in an open
label
fashion. Clinical evaluation was performed weekly with endoscopic
reexamination
performed at the completion of treatment. After therapy, patients were
followed
clinically with endoscopy recommended for recurrent esophageal symptoms.
Of the 12
treated patients, 11 (92%) had a complete symptomatic response; endoscopy
in 11
patients at the completion of treatment showed 9 with complete ulcer
healing, 1
partially healed, and 1 with no response. All responders were asymptomatic
by day 28.
The partial responder received an additional 1 month of thalidomide at 300
mg/day,
resulting in complete endoscopic healing. The patient failing therapy
received
prednisone, but died prior to completing this therapy. On follow-up to 20
months,
six patients have died with no recurrence of IEU. Three patients had
relapse of IEU,
two of whom had a prior history of multiple recurrences of IEU; both of these
patients relapsed within 2 months of completing thalidomide treatment. The
drug was
well tolerated without significant side effects. Thalidomide appears to be
an
effective and well-tolerated alternative to prednisone for the treatment of
IEU.
===================================================================
23. Thalidomide in the treatment of the cutaneous manifestations of lupus erythematosus: experience in sixteen consecutive patients.
===================================================================
Stevens RJ; Andujar C; Edwards CJ; Ames PR; Barwick AR; Khamashta MA;
Hughes GR
Department of Rheumatology, Rayne Institute, St Thomas Hospital, London.
Br J Rheumatol (ENGLAND) Mar 1997 36 (3) p353-9 ISSN: 0263-7103
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9707
Subfile: AIM; INDEX MEDICUS
We review the efficacy, tolerability and safety of low-dose thalidomide
in the
treatment of refractory disfiguring rash in 16 patients with cutaneous
manifestations
of lupus. Rashes, which included discoid lupus erythematosus (DLE), subacute
cutaneous lupus (SCLE), photosensitive malar rash and non-specific chronic
erythema,
were diagnosed on clinical grounds, supported by skin biopsy in 11/16
patients.
Using starting doses of 50-100 mg/day, 7/16 (44%) patients gained complete
or near-
complete remission of skin disease and 6/16 (37%) partial remission. Three
out of 16
patients failed to respond. Maximum benefit was achieved within 16 weeks
in all
patients. Doses of 25-50 mg/day were effective in maintaining response.
Rapid
relapse occurred in 6/8 (75%) patients following drug withdrawal, but the
response to
thalidomide in those requiring repeat courses appeared to be maintained.
There was
no detectable improvement in systemic disease. One patient developed
symptoms of
mild peripheral neuropathy which resolved on drug withdrawal. Our experience
suggests that thalidomide is effective in the treatment of severe skin
manifestations
of lupus refractory to other treatment and can be used safely in specialist
rheumatological practice.
===================================================================
24.) The effect of thalidomide on experimental tumors and metastases.
===================================================================
Minchinton AI; Fryer KH; Wendt KR; Clow KA; Hayes MM
Department of Medical Biophysics, BC Cancer Research Centre, Vancouver,
Canada.
Anticancer Drugs (ENGLAND) May 1996 7 (3) p339-43 ISSN: 0959-4973
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9707
Subfile: INDEX MEDICUS
Thalidomide has recently been shown to antagonize basic fibroblast growth
factor-
induced angiogenesis in the rat corneal micropocket assay. We have
investigated the
effect of thalidomide on growth, radiosensitivity and metastasis in murine
SCCVII and
Lewis Lung tumors. We found that daily thalidomide administration (0.77
mmol/kg/day,
i.p.) does not alter primary tumor growth of SCCVII or Lewis Lung tumors.
However,
thalidomide administration does reduce radiosensitivity of the Lewis Lung
tumor, and
increases its sensitivity to combined treatment with radiation and the
bioreductive
cytotoxin tirapazamine. These findings suggest that thalidomide is
elevating tumor
hypoxia in the Lewis Lung tumor, presumably via an anti-angiogenic
mechanism. We
also found that thalidomide administration reduces the incidence of lung
metastases
from primary Lewis Lung tumors. Thalidomide may therefore have utility in
the
management of solid tumors, especially when combined with drugs that are
selectively
toxic to cells at reduced oxygen tension (e.g. bioreductive cytotoxins).
===================================================================
25.) Paradoxical effect of thalidomide prophylaxis on chronic graft-vs.-hostdisease.
===================================================================
Chao NJ; Parker PM; Niland JC; Wong RM; Dagis A; Long GD; Nademanee AP;
Negrin RS;
Snyder DS; Hu WW; Gould KA; Tierney DK; Zwingenberger K; Forman SJ; Blume KG
Department of Medicine, Stanford University Medical Center, CA 94305, USA.
Biol Blood Marrow Transplant (UNITED STATES) May 1996 2 (2) p86-92
ISSN: 1083-
8791
Language: ENGLISH
Document Type: CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED
TRIAL
Journal Announcement: 9706
Subfile: INDEX MEDICUS
Thalidomide has been reported to be an effective agent for the treatment
of chronic
graft-vs.-host disease (GVHD). To determine its efficacy as a prophylactic
agent for
the prevention of chronic GVHD, a prospective randomized double-blind study
was
performed. A total of 59 patients were randomized to receive either
placebo or
thalidomide (200 mg orally twice a day) beginning 80 days after allogeneic
bone
marrow transplantation (BMT). Fifty-four evaluable patients were analyzed,
26
received placebo, and 28 received thalidomide. The characteristics of
patients were
well-balanced between the two groups. Following the first interim analysis
conducted
by the Data Safety Monitoring Board using an intent-to-treat approach, a
statistically significant difference in the incidence of chronic GVHD was
found.
Patients receiving thalidomide developed chronic GVHD more often than
patients
receiving placebo (p = 0.06). Moreover, an apparent overall survival
advantage was
noted for patients receiving placebo compared to those receiving
thalidomide (p =
0.006). Adjustment for possible confounding factors did not eliminate
these negative
effects of thalidomide. These results demonstrate that while thalidomide
is an
effective agent for the therapy of chronic GVHD, its use at the doses
administered
for the prophylaxis of chronic GVHD resulted in a paradoxical outcome with
a higher
incidence of chronic GVHD and a lower overall survival. We conclude that
the early
use of thalidomide results in a shift in the balance between GVHD and
induction of
tolerance. These data demonstrate again the importance of phase III
double-blind
controlled randomized studies.
===================================================================
26.) Thalidomide and recurrent aphthous stomatitis: a follow-up study.
===================================================================
Bonnetblanc JM; Royer C; Bedane C
Department of Dermatology, CHRU Dupuytren, Limoges, France.
Dermatology (SWITZERLAND) 1996 193 (4) p321-3 ISSN: 1018-8665
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9705
Subfile: INDEX MEDICUS
BACKGROUND: Thalidomide is used for the symptomatic treatment of
recurrent aphthous
stomatitis (RAS). Some authors reported remissions, but this was not
evaluated.
OBJECTIVE: To evaluate the number of patients who could stop or reduce
thalidomide
treatment. METHODS: Twenty-five patients with RAS treated with thalidomide
and
followed during at least 1 year were retrospectively studied. RESULTS: Six
patients
could stop the treatment and further presented minor aphthae, 10 needed
minimal daily
doses of thalidomide and 7 did not respond to 100 mg daily. One patient
was not
evaluated because of an early side effect and one was lost to follow-up.
CONCLUSION:
This study showed that a minority of patients responded and could stop
thalidomide
therapy whereas another group of patients could be maintained in remission
with low
doses of thalidomide which may represent a means to reduce the potentially
severe
side effects.
================================================================
27.) Thalidomide, the product.// FROM THE SKIN THERAPY LETTER
===================================================================
On Friday, September 19, 1997 the FDA indicated to the Celgene
Corporation that thalidomide, Thalomid® has been designated as
approvable (see explanation below) for the treatment of cutaneous
manifestations of erythema nodosum leprosum (ENL). In this condition
there are no good alternative treatments to thalidomide. ENL is a
severe and painful complication for approximately half of all leprosy
patients, affecting about two million people worldwide.
The Thalidomide Chronology
Introduced in the late 1950's
First marketed as a sedative & for morning sickness. Never
marketed in the US.
Worldwide ban in 1961
Associated with phocomelia & other congenital abnormalities.
Sheskin, 19651
Found thalidomide effective in erythema nodosum leprosum. Dose
of 100 mg three to four times daily.
From the ban until now
Unapproved, illegal use. Bootleg/blackmarket supply.
Compassionate use approval available in the US and Canada.
Supplies
from Carville, La. in the past, Celgene now.
1988 - WHO recommend
WHO's treatment of choice for severe ENL. Based on the results of
a double-blind, multi-centered trial.
Late 1997 - first US approval nears
FDA Advisory Panel September 5, 1997 recommends the approval of
thalidomide for ENL.
FDA, September 19, 1997 designates thalidomide as approvable for
the treatment of cutaneous manifestations of ENL.
Future indications
Candidates are AIDS-related cachexia or aphthous ulcers,2
graft versus
host disease, and recalcitrant discoid lupus erythematosus.
Future developments
Celgene and Andrulis are involved in on-going clinical trials
with
thalidomide. Celgene are working on developing related
compounds with
useful activity and fewer side effects.
Use of thalidomide for ENL
The WHO has stated that thalidomide is a treatment of choice for
severe
ENL and now the FDA has decided that the benefits of treatment with
thalidomide outweigh the risks involved. ENL can be life
threatening and
may cause permanent nerve paralysis and disfigurement. Previously
available treatments for severe ENL, corticosteroids and
clofazimine are
not very effective. Mild ENL has been successfully treated with
aspirin,
indomethacin, chloroquine or colchicine.
If Celgene's claim that at least 90% of ENL patients respond to
thalidomide proves correct, does this raise the possibility of
thalidomide
being used for all cases of ENL?
Dr. Stuart Maddin, Editor
Other uses of thalidomide
More than 20 clinical trials are underway and the drug is also
supplied on an
emergency use basis or investigator IND basis for over 30
conditions. Currently
the dermatologic conditions include Behcet's disease, prurigo
nodularis, discoid
lupus erythematosus, pyoderma gangrenosum, erythema multiforme,
Jessner's
lymphatic infiltration, pompholyx, scleroderma, urticaria, bullous
pemphigoid
and cutaneous sarcoidosis.3
Mechanism of action
The mechanism by which thalidomide reduces the elevated levels of
tumor
necrosis factor-alpha (TNF-alpha) associated with ENL is yet to be
understood.4 Thalidomide has other immunopharmacologic actions
which are
under investigation.4
Thalidomide prevents the immune system from overreacting to disease
and harming the body. Among its known modes of action is the
inhibition
of production of cytokine TNF-alpha.
Dr Kaplan, Rockefeller University.5
Precautions Restricted distribution Celgene designed and has submitted a
restricted distribution proposal (System for Thalidomide Education and
Prescribing Safety (S.T.E.P.S.) to the FDA. The objective of the S.T.E.P.S. program is
to help insure that fetal exposure to thalidomide occurs with the lowest
possible incidence. This comprehensive program will be directed to physicians,
pharmacists, and patients, both male and female. It will require all physicians
and pharmacies to register in order to prescribe or dispense Thalomid™ (thalidomide) and all patients to complete an informed consent process and
participate in a mandatory and confidential surveillance registry.
There are precedents for restricted distribution of a drug,
isotretinoin,clozapine and fentanyl oralet have all been marketed successfully this
way, and thalidomide when approved, will have more restrictions on it than any drug ever sold in the U.S.
M. Lumpkin, FDA's Centre for Drug Evaluation6
Off-label use Some members of the FDA's Advisory panel have suggested
that off-label use for other illnesses be prohibited; however the
distribution system is designed to cover any use of the drug.
"We need to make this system as leak-proof as possible."
Dr. J. McGuire, Stanford
(Advisory Committee Chairman)7
Informed consent waivers Patients and physicians will have to sign detailed
informed consent waivers.
Contraception Female patients will have to agree to use two forms
of birth control, to undergo ongoing pregnancy tests and to participate in
monthly surveys. Patients who have irregular menstrual periods, vaginal
bleeding or missed periods may need more frequent pregnancy tests. Males will
have to agree to use condoms and to complete surveys every three months.
They must abstain from sexual intercourse or use a condom during intercourse
while, and for one month after, taking thalidomide. It is not known if thalidomide
is present in semen.8
A female patient must immediately stop taking thalidomide if she:
Has a late or irregular period.
Stops practicing abstinence.
Stops using birth control.
Thinks that she is pregnant.
Does become pregnant.9
Supply of thalidomide
Thalomid™ will possibly be available commercially in the first half of
this year. The projected cost of a 50 mg capsule is US$6, meaning that daily
treatment of ENL (100-200 mg per day) will cost approximately US$12-24. Patients
will only get a 28 day supply; subsequent supply requires a new prescription.
Adverse effects
The most common adverse effects are drowsiness, rash and constipation.
Peripheral neuropathy
Peripheral neuropathy occurs in less than 1% of ENL patients
treated with thalidomide, despite long-term treatment, pre-existing
neuropathies, or use in patients who are receiving other medications known to be associated with neuropathies.3 Neuropathy is more common (between 21% and 50%) in AIDS
patients. The neuropathy generally occurs following chronic use over
a period of months but reports following relatively short-term use also exist. In
some cases, the nerve damage has proved irreversible even after treatment with
the drug is discontinued. Individual susceptibilities, with possible genetic predisposition, seem to be more important than daily dose and duration of
therapy.10 Patients should be examined for early signs of neuropathy at monthly
intervals for the first three months.3 Symptoms of nerve damage include numbness,
tingling or pain in the arms, hands, legs and feet. Patients should be warned
of this side effect, and understand that they must stop thalidomide immediately if
paresthesias develop.11 To detect asymptomatic neuropathy, consider
measuring sensory nerve action potential (SNAP) at baseline and every six
months. If symptoms develop, stop thalidomide immediately and only restart
therapy if the neuropathy completely resolves.
Birth defects
At the meeting of the FDA Advisory Committee, a spokesman for the5,000
individuals with birth defects caused by thalidomide who are still
living, was saddened at the prospect of potential approval but said that the
group preferred regulation to unmonitored use of black market supplies. Celgene said
that there have been no birth defects reported so far among the 5,000 ENL
patients who have received thalidomide, either through clinical trials or on an
emergency basis.
Professor Louis Dubertret of Paris is of the opinion that the French
regulatory controls for distribution and use have made thalidomide a very safe
drug for the very limited number of patients receiving it.
Future Possibilities
Molecular manipulation has uncovered other thalidomide-related compounds
which inhibit TNF-alpha production more efficiently than
thalidomide, and cause fewer side effects in animals.5 Celgene's first compound entered Phase1 clinical study in 1997.3 The goal, a non-teratogenic compound, with equal or
greater immunomodulating potential than thalidomide, offers the
exciting possibility of new and relatively safe compounds which may prove effective in treating diseases at present resistant to currently available therapies. Thalidomide itself has a range of interesting and potentially useful immuno-pharmacologic actions4 and after further study and sensible precautions as to its use, has a clear potential as a future immunomodulator.
References
1.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Phar
Therap 1965; 6: 303.
2.Jacobsen JM, Greenspan JS, Spritzler J et al. Thalidomide for the
treatment of oral aphthous ulcers in patients with human
immunodeficiency virus infection. N Engl J Med 1997; 336: 1487-1493.
3.David Stirling, Celgene. Personal communication. January, 1997.
4.Calderon P, Anzilotti M, Phelps R. Thalidomide in dermatology. New
indications for an old drug. Int J Dermatol 1997; 36: 881-887.
5.Kaplan G. quoted by Blaney C., Second thoughts about thalidomide.
Medical Sciences Bulletin, originally published in NCRR Reporter,
November/December 1995.
6.Lumpkin M., Deputy Director FDA's Center for Drug Evaluation
speaking at a meeting of the FDA Advisory Committee, quoted in
Reuters Medical News.
7.McGuire J. Personal communication. October, 1997.
8.Thalidomide: Important patient information. US FDA Center for Drug
Evaluation and Research.
http://www.fda.gov/cder/news/thalidomide.htm.
9.Burkholz H. Giving thalidomide a second chance. FDA Consumer
Magazine 1997: September-October.
10.Ochonisky SO, Verroust J, Basuji-Garin S. et al. Arch Dermatol 1994;
130: 66-69.
11.Powell RJ, Garner-Medwin JMM. Postgrad Med J 1994; 70: 901-904.
12.Dubertret L. Personal communication. October, 1997.
Approvable Status: An approvable letter indicates that FDA is prepared
to approve the application upon the satisfaction of conditions specified in
the approvable letter. Such drug products may not be legally marketed
until the firm has satisfied the identified deficiencies, as well as any other
requirements that may be imposed by the FDA, and has been notified in
writing that the application has been approved.
===========================================================
28.) FDA Clears Thalidomide For Leprosy
===========================================================
WARREN, NJ -- July 16, 1998 - The United States Food and Drug
Administration has granted marketing clearance to Celgene Corp.'s
Thalomid(TM) (thalidomide) for the treatment of erythema nodosum leprosum
(ENL), a severe and debilitating condition associated with leprosy.
Celgene licensed rights to thalidomide from The Rockefeller University in 1992
and began developing the drug for a range of potential indications. These
include AIDS related, dermatological and cancer related conditions.
In order to support the safe and appropriate use of the drug, due to concern
about the teratogenic potential of thalidomide in humans when the drug is take
during pregnancy, Celgene has developed a unique and comprehensive patient,
physician and pharmacist education and distribution system to be called the
System for Thalidomide Education and Prescribing Safety (STEPS).
Major Components of the STEPS Program include:
-- Physicians prescribing Thalomid and pharmacists dispensing the drug will
register and receive educational materials explaining risks and pregnancy
prevention methods, as well as expected side effects of therapy.
-- All females who are candidates for Thalomid therapy will be required to
undergo pregnancy testing before starting treatment and periodically thereafter.
-- Women will also be required to use effective birth control while taking the
drug.
-- Men using the drug will be required to use condoms when having sexual
relations with women.
-- All candidates for Thalomid therapy will be provided with counselling by the
physician and comprehensive multicultural and multilingual educational material
clearly explaining the risks of use.
-- Patients will be required to sign an informed consent form after an
explanation of the risks of Thalomid use by the physician.
-- Before a prescription is filled, a copy of the informed consent form st be
presented at a pharmacy pre-registered to dispense Thalomid.
-- Persons using Thalomid will be required to participate in a patient survey
designed to determine compliance with the STEPS program and any situations
involving pregnancy.
-- Patients will be instructed never to share the drug with other persons,
including friends or relatives with the same symptoms for which Thalomid was
prescribed.
-- Thalomid packaging will contain clear and prominent warnings about the
risks associated with use. Prescriptions for Thalomid will be no more than a 28
day supply with no automatic renewals.
======================================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No (8) 23/10/98 DR. JOSE LAPENTA R. DERMATÓLOGO
======================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.024
Tlf: 0414-2976087 - 04127766810

















.png)