LA PENICILAMINA, Cuprimine
La D-penicilamina es la forma dextro de un compuesto formado a partir de la hidrólisis de la penicilina. Por su capacidad para unirse a metales pesados y facilitar su excreción, así como por sus propiedades antiinflamatorias. Nombre comercial: CUPRIMINE.
Es un agente quelante, lo que significa que es capaz de unirse a metales pesados para eliminarlos del cuerpo, (sobre todo el COBRE) , por lo tanto es útil en enfermedades como:
1.) La enfermedad de WILSON: condición genética que provoca la acumulacion de cobre en el cuerpo, y este medicamento al unirse con el metal lo elimina del cuerpo.
2.) Intoxicación con Metales pesados como: PLOMO y MERCURIO.
3.) Cistinuria: Trastorno genético que provoca la generación de cálculos renales de cistina, en donde esta medicina reduce la formación de los mismos.
4.) Artritis reumatoidea: cuando no responde a los tratamientos clásicos, sobre todo en la asociada a enfermedades autoinmunes.
5.) En dermatología la D-Penicilamina se utiliza en enfermedades autoinmunes específicamente la ESCLERODERMIA, o ESCLEROSIS SISTÉMICA, también en la morfea Profunda.
6.) Otras enfermedades donde ha sido utilizada: Cáncer de hígado y enfermedades inflamatorias autoinmunes.
Esta medicina tiene efectos secundarios SEVEROS, tanto así que en el empaque tiene lo que llaman un "BLACK BOX" o caja negra que es una advertencia tanto para el médico como los pacientes del delicado uso de esta medicina.
Entre estos efectos secundarios destacan: daño renal (nefrotoxicidad), gastrointestinales (náuseas y vómitos), hematológicos (trombocitopenia), y reacciones alérgicas.
LA CAJA NEGRA del empaque dice literalmente lo siguiente:
"Los médicos que planeen usar penicilamina deben familiarizarse completamente con su toxicidad, consideraciones de dosis especiales y beneficios terapéuticos. La penicilamina nunca debe usarse de manera casual. Cada paciente debe permanecer constantemente bajo la estrecha supervisión del médico.
Se debe advertir a los pacientes que informen de inmediato sobre cualquier síntoma que sugiera toxicidad."
Saludos,,,
Dr. José Lapenta.
ENGLISH
D-penicillamine is the dextro form of a compound formed from the hydrolysis of penicillin. For its ability to bind to heavy metals and facilitate their excretion, as well as for its anti-inflammatory properties. Trade name: CUPRIMINE.
It is a chelating agent, which means that it is able to bind to heavy metals to eliminate them from the body, (especially COPPER), therefore it is useful in diseases such as:
1.) WILSON'S disease: genetic condition that causes the accumulation of copper in the body, and this medicine by binding with the metal eliminates it from the body.
2.) Poisoning with heavy metals such as: LEAD and MERCURY.
3.) Cystinuria: Genetic disorder that causes the generation of cystine kidney stones, where this medicine reduces the formation of them.
4.) Rheumatoid arthritis: when it does not respond to classic treatments, especially in cases associated with autoimmune diseases.
5.) In dermatology, D-Penicillamine is used in autoimmune diseases, specifically SCLERODERMA, or SYSTEMIC SCLEROSIS, and also in Deep Morphea.
6.) Other diseases where it has been used: Liver cancer and autoimmune inflammatory diseases.
This medicine has SEVERE side effects, so much so that on the packaging it has what they call a "BLACK BOX" which is a warning for both the doctor and the patients of the delicate use of this medicine.
Among these side effects are: kidney damage (nephrotoxicity), gastrointestinal (nausea and vomiting), hematological (thrombocytopenia), and allergic reactions.
THE BLACK BOX on the packaging literally says the following:
"Physicians planning to use penicillamine should become thoroughly familiar with its toxicity, special dosage considerations, and therapeutic benefits. Penicillamine should never be used casually. Each patient should remain constantly under the close supervision of the physician.
Patients should be warned to immediately report any symptoms suggesting toxicity."
Greetings...
Dr. José Lapenta R.
EDITORIAL ESPANOL:
====================
Hola amigos de la red, DERMAGIC con ustedes de nuevo. El tema de hoy, la D-PENICILAMINA, medicamento muy utilizado en enfermedades del colágeno (esclerosis sistémica, esclerodermia localizada, etc), con efectos beneficiosos en algunos casos y en otros con efectos adversos. Estas 52 referencias nos permiten conocer lo bueno y malo de este producto. Al final una monografía del Cuprimine.
PRÓXIMAS EDICIONES: * El síndrome de Raynaud * Lupus eritematoso discoide.
Saludos,,,
Dr. José Lapenta R.,,,
EDITORIAL ENGLISH:
===================
Hello friends of the net, DERMAGIC with you again. Today's topic, the D-PENICILAMINA, medication very used in the connective tissue diseases (systemic sclerosis, localized scleroderma), with beneficial effects in some cases and in others with adverse effects. These 52 references allow us to know the good and bad of this product. At the end a monograph of the Cuprimine.
NEXT EDITIONS: The Raynaud's Syndrome. Discoid lupus erythematosus.
Greetings,,,
Dr. José Lapenta R.
=======================================================================
DERMAGIC/EXPRESS(50)
=======================================================================
LA D-PENICILAMINA / THE D-PENICILLAMINE
=======================================================================
1.) A 15-year prospective study of treatment of rapidly progressive systemic
Sclerosis with D-penicillamine [see comment]
2.) Prevention of acute autoimmune encephalomyelitis and abrogation of relapses
in murine models of multiple sclerosis by the protease inhibitor D-penicillamine.
3.) Treatment of systemic sclerosis with extracorporeal photochemotherapy.
Results of a multicenter trial [see comments]
4.) copper chelator d-penicillamine delays onset of disease and extends
survival in a transgenic mouse model of familial amyotrophic lateral
sclerosis.
5.) D-Penicillamine in systemic sclerosis: a reappraisal.
Author
6.) Treatment of systemic sclerosis.
7.) D-penicillamine treatment of progressive systemic sclerosis (scleroderma): a comparison of clinical and in vitro effects.
8.) D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis.
9.) Successful treatment of far-advanced progressive systemic sclerosis by D-penicillamine.
10.) Does long-term treatment with D-penicillamine alter calcium and phosphorus metabolism in patients with systemic sclerosis?
11.) 65Zinc absorption in untreated and D-penicillamine-treated patients with generalized scleroderma: determination by whole-body counting technique.
12.) Changes in the growth and metabolism of cells cultured from normal, sclerotic and rheumatoid connective tissue brought about by D-penicillamine and by sodium salicylate.
13.) Inhibition of neutrophil oxidant secretion by D-penicillamine: scavenging of H2O2 and HOCl.
14.) Lack of induction of antinuclear antibodies by D-penicillamine in rheumatoid arthritis: a controlled study.
15.) Influence of food on the bioavailability of penicillamine.
16.) Treatment of generalized scleroderma with inhibitors of collagen synthesis.
17.) Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis.
18.) Intermittent treatment with D-penicillamine is effective in lower doses and with fewer adverse effects in patients with rheumatoid arthritis.
19.) A western blot approach to detection of human plasma protein conjugates derived from D-penicillamine.
20.) Disease-modifying antirheumatic drugs, including methotrexate, sulfasalazine, gold, antimalarials, and D-penicillamine.
21.) Frequent induction of insulin autoantibodies by D-penicillamine in patients with rheumatoid arthritis.
22.) The fasciitis-morphea complex in children.
23.) The interactions of penicillamine with copper in vivo and the effect on hepatic metallothionein levels and copper/zinc distribution: the implications for Wilson's disease and arthritis therapy.
24.) Comparison of cyclosporine and D-penicillamine for rheumatoid arthritis: a randomized, double blind, multicenter study.
25.) D-penicillamine in the treatment of localized scleroderma [see coms]
26.)Inhibition of human endothelial cell proliferation in vitro and neovascularization in vivo by D-penicillamine.
27.) [Pemphigus herpetiformis following D-penicillamine in a patient with HLA B8]
28.) Progressive systemic sclerosis complicated with immune thrombocytopenia
during D-penicillamine therapy.
29.) Lupus erythematosus-like eruption due to D-penicillamine in progressive
systemic sclerosis.
30.) Bronchiolitis obliterans in a patient with localized scleroderma treated
with D-penicillamine.
31.) [Extracapillary glomerulonephritis secondary to D-penicillamine. Apropos of
1 case and review of the literature]
32.) Unilateral ptosis as an initial manifestation of D-penicillamine induced
myasthenia gravis.
33.) C1 inhibitor deficiency in a patient with rheumatoid arthritis--increased
risk of adverse effects of penicillamine?
34.) D-penicillamine- and quinidine-induced antinuclear antibodies in A.SW
(H-2s) mice: similarities with autoantibodies in spontaneous and heavy
metal-induced autoimmunity.
35.) Combination therapy for rheumatoid arthritis and drug-induced systemic
lupus erythematosus.
36.) Chronic modulation of the autoimmune response following parvovirus B19
infection.
37.) Massive breast enlargement in a patient receiving D-penicillamine for systemic sclerosis.
38.) Progressive systemic sclerosis complicated with immune thrombocytopenia during D-penicillamine therapy.
39.) Systemic sclerosis-like lesions during long-term penicillamine therapy for Wilson's disease.
40.) The toxicity of D-penicillamine in systemic sclerosis.
41.) D-penicillamine induced crescentic glomerulonephritis: report and review of the literature.
42.) Antibodies to cellular antigens in Greek patients with autoimmune rheumatic diseases: anti-Ro(SSA) antibody a possible marker of penicillamine-D intolerance.
43.) HLA system and side effects of gold salts and D-penicillamine treatment of rheumatoid arthritis.
44.) In vitro inhibition of myelopoiesis by gold salts and D-penicillamine.
45.) Autoimmune associations in primary biliary cirrhosis.
46.) Sequential histopathologic alterations in Indian childhood cirrhosis treated with d-penicillamine [see comments]
47.) Pure red cell aplasia caused by D-penicillamine treatment of rheumatoid arthritis.
48.) A case of bullous pemphigoid induced by tiobutarit (D-penicillamine analogue).
49.) Fatal aplastic anaemia and liver toxicity caused by D-penicillamine treatment of rheumatoid arthritis.
50.) D-penicillamine-induced elastosis perforans serpiginosa in a child with juvenile rheumatoid arthritis. Report of a case and review of the literature.
51.) Nodular scleroderma in systemic sclerosis under D-penicillamine therapy.
52.) D-PENICLLAMINE, the product
=======================================================================
=======================================================================
1.) A 15-year prospective study of treatment of rapidly progressive systemic
Sclerosis with D-penicillamine [see comment]
=======================================================================
Author
Jimenez SA; Sigal SH
Address
Department of Medicine, Jefferson Medical College, Thomas Jefferson
University, Philadelphia, PA 19107.
Source
J Rheumatol, 18(10):1496-503 1991 Oct
Abstract
A 15-year (1973-1988) prospective study was conducted to determine the
effectiveness of D-penicillamine (D-pen) in the treatment of rapidly
progressive systemic sclerosis (SSc) of recent onset. Sixty-nine
consecutive patients fulfilling strict criteria for rapidly progressive
diffuse SSc of less than 18 months of duration were enrolled. Sixty
received at least 750 mg/day of D-pen for at least 6 months, whereas 9 did
not complete 6 months of treatment because of toxicity, noncompliance or
death. In 58 of the 60 patients treated for longer than 6 months, there was
an arrest in the progression of skin sclerosis followed by regression with
softening, increased pliability and reappearance of sweating and hair. In
these cases, the extent of sclerotic skin decreased from a maximum of 64.6
+/- 23.1% total body surface to 15.7 +/- 13.2%. In addition, in the group
of patients that received D-pen for longer than 6 months, SSc renal disease
was uncommon and pulmonary involvement was not progressive. The overall
survival in this group was 88.3%. In conclusion, our prospective study
showed that the administration of D-pen resulted in significant improvement
of skin sclerosis and in prolonged survival of patients with early, rapidly
progressive SSc with diffuse cutaneous involvement.
=======================================================================
2.) Prevention of acute autoimmune encephalomyelitis and abrogation of relapses
in murine models of multiple sclerosis by the protease inhibitor D-penicillamine.
=======================================================================
Author
Norga K; Paemen L; Masure S; Dillen C; Heremans H; Billiau A; Carton H;
Cuzner L; Olsson T; Van Damme J; et al
Address
Laboratory of Molecular Immunology, University of Leuven, Belgium.
Source
Inflamm Res, 44(12):529-34 1995 Dec
Abstract
The in vitro activity of gelatinase B, an enzyme whose appearance in the
cerebrospinal fluid is associated with inflammatory diseases of the central
nervous system, was dose-dependently inhibited by the antirheumatic
D-penicillamine. Inhibition of gelatinase B in electrophoretically pure
preparations and in cell culture supernatants and human body fluids was
obtained at dosages reached in the circulation of patients treated with a
peroral dosis of 750 mg D-penicillamine per day. In mice, developing acute
demyelination, D-penicillamine significantly reduced the mortality and
morbidity rates of experimental allergic encephalomyelitis (EAE). In
chronic relapsing EAE in Biozzi AB/H mice, an animal model for relapses in
multiple sclerosis (MS), it attenuated the exacerbations, even when the
treatment was started after the primary full-blown disease had developed.
We infer protease inhibition as the mechanism of action of D-penicillamine
and suggest that its use may be effective as peroral treatment for MS.
=======================================================================
3.) Treatment of systemic sclerosis with extracorporeal photochemotherapy.
Results of a multicenter trial [see comments]
=======================================================================
Author
Rook AH; Freundlich B; Jegasothy BV; Perez MI; Barr WG; Jimenez SA;
Rietschel RL; Wintroub B; Kahaleh MB; Varga J; et al
Address
Department of Dermatology, Hospital of the University of Pennsylvania,
Philadelphia 19104.
Source
Arch Dermatol, 128(3):337-46 1992 Mar
Abstract
BACKGROUND AND DESIGN--In a pilot study of extracorporeal
photochemotherapy, two patients with systemic sclerosis who received this
therapy experienced significant clinical improvement. These results
prompted the development of a multicenter trial to examine the benefit of
extracorporeal photochemotherapy in the treatment of systemic sclerosis.
Seventy-nine patients with systemic sclerosis of recent onset (mean symptom
duration, 1.83 years) and progressive skin involvement during the preceding
6 months entered a randomized, parallel-group, single-blinded clinical
trial comparing extracorporeal photochemotherapy treatments given on 2
consecutive days monthly with treatment with D-penicillamine at a maximum
dose of 750 mg/d. Blinded clinical examiners evaluated skin severity score
(thickness), percent surface area involvement, oral aperture, and hand
closure. Serial skin biopsies and pulmonary function studies were also
performed. RESULTS--Following 6 months of treatment, significant
improvement in skin severity score occurred in 21 (68%) of 31 patients
receiving photochemotherapy and in eight (32%) of 25 receiving
D-penicillamine treatment, while significant worsening occurred in three
(10%) of 31 receiving photochemotherapy and in eight (32%) of 25 receiving
penicillamine treatment, thus indicating a significantly higher response
rate for individuals who received photochemotherapy (P = .02). At both the
6- and 10-month evaluation points, the mean skin severity score, mean
percent skin involvement, and mean oral aperture measurements were
significantly improved from baseline among those who received
photochemotherapy. Mean right and left hand closure measurements had also
improved significantly by 10 months of therapy. By comparison, among the
patients treated with D-penicillamine, none of the parameters of cutaneous
disease had improved significantly after 6 months of therapy, although for
those individuals in whom treatment was continued, the mean skin severity
score and mean percent skin involvement had improved by 10 months. Skin
biopsy studies revealed a correlation between clinical improvement and
decreased thickness of the dermal layer. Adverse effects of extracorporeal
photochemotherapy were minimal and did not require discontinuation of
treatment in any of the patients receiving this therapy; six patients
permanently discontinued the use of D-penicillamine treatment due to
adverse effects. CONCLUSIONS--For patients with systemic sclerosis of
recent onset, extracorporeal photochemotherapy is a well-tolerated
treatment that may partially reverse the process that results in cutaneous
sclerosis.
=======================================================================
4.) copper chelator d-penicillamine delays onset of disease and extends
survival in a transgenic mouse model of familial amyotrophic lateral
sclerosis.
=======================================================================
Author
Hottinger AF; Fine EG; Gurney ME; Zurn AD; Aebischer P
Address
Gene Therapy Center, Lausanne University Medical School, Switzerland.
Source
Eur J Neurosci, 9(7):1548-51 1997 Jul
Abstract
A subpopulation of familial cases of amyotrophic lateral sclerosis has been
linked to mutations in the gene encoding Cu/Zn superoxide dismutase (SOD1).
There is in vitro evidence that certain SOD1 mutants, in addition to their
normal dismutation function, show increased ability of the enzyme to act as
a peroxidase. This reaction is sensitive to inhibition by copper chelators.
To test this hypothesis in vivo, we administered the copper chelator
d-penicillamine to a transgenic mouse model of familial amyotrophic lateral
sclerosis overexpressing a mutated form of human SOD1. We demonstrate that
oral administration of d-penicillamine is able to delay the onset of the
disease and extend the survival of these mice. Histological studies also
showed a decreased loss of facial motor neurons in d-penicillamine-treated
transgenic mice, corroborating the slower evolution of the disease in these
animals. These results suggest that copper chelators may benefit patients
with familial amyotrophic lateral sclerosis linked to mutations in the SOD1
gene.
=======================================================================
5.) D-Penicillamine in systemic sclerosis: a reappraisal.
Author
=======================================================================
Sattar MA; Guindi RT; Sugathan TN
Address
Department of Medicine, Faculty of Medicine, Kuwait University.
Source
Clin Rheumatol, 9(4):517-22 1990 Dec
Abstract
In a 36-month prospective trial 21 patients with systemic sclerosis
(diffuse systemic sclerosis 16 patients and 5 subjects with limited
cutaneous subtype) were treated with D-penicillamine. In all patients with
diffuse systemic sclerosis there was objective improvement. The degree and
extent of skin involvement decreased significantly (p less than 0.001),
whereas no objective improvement was noted in patients with limited
cutaneous subtype. Further, no systemic progression of the disease was
observed during the study period. Our results suggest that a prolonged
treatment with D-penicillamine in small doses is not only beneficial and
effective but also free of side-effects, if used at an earlier stage.
=======================================================================
6.) Treatment of systemic sclerosis.
=======================================================================
Author
Wigley FM
Address
Johns Hopkins University School of Medicine, Baltimore, Maryland.
Source
Curr Opin Rheumatol, 4(6):878-86 1992 Dec
Abstract
A new awareness of the challenges and pitfalls of clinical research in
patients with systemic sclerosis has recently arisen. Several editorials
discussed concern about the design of therapeutic trials and the need to
use established scientific standards to find better markers of disease
activity and better ways to measure improvement or deterioration of organ
involvement, including the heart, lung, and gastrointestinal tract. This
year, an uncontrolled experience in the use of D-penicillamine in the
treatment of patients with rapidly progressive skin involvement was
reported. In addition, a multicenter study of photopheresis demonstrated
benefit compared with D-penicillamine. Several new prokinetic drugs
demonstrated promise for the treatment of gastrointestinal disease in
patients with systemic sclerosis. Although studies continue to demonstrate
the benefit of intravenous prostaglandins in the treatment of Raynaud's
phenomenon and digital ulcers in scleroderma, an initial report of oral
prostaglandins was disappointing. Clinical researchers are now working
together to design multicenter studies and to define new uniform standards
of disease activity so that the appropriate treatment for systemic
sclerosis can be determined.
=======================================================================
7.) D-penicillamine treatment of progressive systemic sclerosis (scleroderma): a comparison of clinical and in vitro effects.
=======================================================================
SO - J Rheumatol 1983 Apr;10(2):316-8
AU - Shapiro LS; Prince RK; Buckingham RB; Rodnan GP
MJ - Penicillamine [therapeutic use]; Scleroderma, Systemic [drug therapy]
MN - Adult; Aged; Cells, Cultured; Fibroblasts [metabolism]; Glycosaminoglycans [metabolism]; Middle Age; Scleroderma, Systemic [metabolism] [pathology]
MT - Female; Human; In Vitro; Support, Non-U.S. Gov't; Support, U.S. Gov't, P.H.S.
PT - JOURNAL ARTICLE
AB - Punch biopsies of skin were obtained from the forearms of 3 patients with progressive systemic sclerosis with diffuse scleroderma before and after treatment for 1 year or more with D-penicillamine. While on therapy, each patient studied had demonstrated a marked reduction in skin thickening. Collagenase-sensitive protein and glycosaminoglycan accumulation were measured in fibroblast cultures derived from these biopsies. No differences were observed pre- and post-treatment. We conclude that although D-penicillamine may exert its effect in vivo on connective tissue synthesis, maturation and/or turnover, fibroblasts remaining in the thinned dermis retain their potential for increased synthesis of connective tissue.
=======================================================================
8.) D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis.
=======================================================================
SO - Ann Intern Med 1982 Nov;97(5):652-9
AU - Steen VD; Medsger TA Jr; Rodnan GP
PT - JOURNAL ARTICLE
AB - In a retrospective study on progressive systemic sclerosis, we compared 73 patients who had received D-penicillamine therapy for a minimum of 6 consecutive months with 45 patients who had not received this drug. All patients had diffuse sclerodermatous skin changes and early disease (less than 3-years duration). D-Penicillamine was prescribed for an average of 24 months (range, 6 to 68 months) with a maximum daily dose of 500 to 1500 mg (median, 750 mg). During a mean follow-up interval of 38 months, the degree and extent of skin thickness, determined on physical examination, decreased considerably more in the patients treated with D-penicillamine than in patients in the comparison group (p = 0.07). The rate of new visceral organ involvement was reduced in patients treated with D-penicillamine, especially for the kidney (p = 0.01). Patients treated with D-penicillamine had a greater 5-year cumulative survival rate (88% versus 66%, p less than 0.05). Therapy with colchicine (23 patients) or immunosuppressive agents (26 patients) was not associated with these improvements.
=======================================================================
9.) Successful treatment of far-advanced progressive systemic sclerosis by D-penicillamine.
=======================================================================
SO - J Allergy Clin Immunol 1982 Mar;69(3):297-305
AU - Kang B; Veres-Thorner C; Heredia R; Cha E; Bose S; Schwartz M
PT - JOURNAL ARTICLE
AB - A 52-yr-old man had chronic recurrent pseudo-obstruction of the colon, along with multisystem disease secondary to progressive systemic sclerosis (PSS). D-Penicillamine treatment reversed the PSS, which involved the duodenum, small and large intestines, lungs, myocardium, and proximal and peripheral skin. The improvement was documented by electrocardiogram pulmonary function studies, roentgenography of gastrointestinal tract, and by repeated skin biopsies. Hand blood-volume studies using 99m Tc also revealed improvement of PSS in both hands. A variable degree of improvement was documented in seven additional patients with PSS. Follow-up periods were 18 mo to 4 yr. The most common side effect of the drug was transient hypogeusia, and no patient showed a serious side effect. This article includes a review of the literature on penicillamine therapy for PSS.
=======================================================================
10.) Does long-term treatment with D-penicillamine alter calcium and phosphorus metabolism in patients with systemic sclerosis?
=======================================================================
SO - Acta Derm Venereol 1983;63(5):447-9
AU - Hagdrup H; Serup J; Tvedegaard E
PT - JOURNAL ARTICLE
AB - Only animal experiments have been published regarding the possible effect of penicillamine treatment on mineral balance. We examined 35 patients with a diagnosis of systemic sclerosis treated with penicillamine, in comparison with 10 patients with systemic sclerosis treated with other collagen inhibitors (glutamine, hydralazine, phenytoin, chlorpromazine). The following laboratory tests were performed in all patients: the serum concentrations of calcium, ionized calcium, phosphorus and the parathyroid hormone. The 24-hour urinary excretions of calcium and phosphorus were determined. The bone mineral content of the distal radius was determined by photon-absorptiometry; radiological examination of the hands was performed to show aberrant calcifications. No differences were found between the two groups. A low urinary excretion of calcium and phosphor was found in the entire material. The bone mineral content was low in both groups. A high frequency of aberrant calcifications was not correlated to treatment with penicillamine.
=======================================================================
11.) 65Zinc absorption in untreated and D-penicillamine-treated patients with generalized scleroderma: determination by whole-body counting technique.
=======================================================================
SO - Acta Derm Venereol 1982;62(5):438-40
AU - Hoyer H; Hvid-Jacobsen K; Weismann K
PT - JOURNAL ARTICLE
AB - 65Zinc absorption in patients suffering from generalized scleroderma was studied by means of whole-body counting technique following a single dose of 65Zn. In 4 untreated patients the mean 65Zn absorption was calculated to 35% (range 20-59%). Five patients receiving oral D-penicillamine had a numerically higher mean absorption value of 55% (range 37-74%). The results corroborate earlier studies on the effect of D-penicillamine on 65Zn absorption in rats.
=======================================================================
12.) Changes in the growth and metabolism of cells cultured from normal, sclerotic and rheumatoid connective tissue brought about by D-penicillamine and by sodium salicylate.
=======================================================================
SO - J Invest Dermatol 1980 Jun;74(6):413-7
AU - Priestley GC
MT - Human; In Vitro
PT - JOURNAL ARTICLE
AB - D-penicillamine and sodium salicylate were equally effective in suppressing the proliferation of fibroblasts from normal and scleroderma skin and rheumatoid synovial cells. The effects were concentration-dependent, beginning at around 100 microgram/ml, and proliferation was almost halted at 1600 microgram/ml. Individual cell strains of each type showed differences in drug susceptibility but there was no consistent difference between cells from normal and abnormal tissues. Acid mucopolysaccharide secretion was more clearly inhibited in scleroderma fibroblasts than in synovial cells, and penicillamine produced greater inhibition than salicylate, beginning at 10 microgram/ml and reaching almost 50% at 500 microgram/ml. Confluent cultures of scleroderma fibroblasts showed 23% less incorporation of 3H-proline into collagenase-sensitive protein in the presence of 1600 microgram/ml penicillamine, without significant effect on other protein synthesis; sodium salicylate had no effect. These data suggest that D-penicillamine may affect connective tissue metabolism in other ways than by interfering with collagen synthesis or cross-linking.
=======================================================================
13.) Inhibition of neutrophil oxidant secretion by D-penicillamine: scavenging of H2O2 and HOCl.
=======================================================================
SO - Ann Rheum Dis 1992 Mar;51(3):321-5
AU - Ledson MJ; Bucknall RC; Edwards SW
AD - Department of Biochemistry, University of Liverpool, United Kingdom.
PT - JOURNAL ARTICLE
AB - D-Penicillamine inhibited oxidant secretion from human neutrophils after activation by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMet-Leu-Phe), as assessed by luminol dependent chemiluminescence. In contrast, this drug had little effect on either intracellular oxidant production or lucigenin dependent chemiluminescence activated by the same agonist. The drug was shown to scavenge both H2O2 and HOCl in a cell free luminol chemiluminescence system, though its ability to scavenge HOCl was greater than that for H2O2. Both these oxidants could oxidise the drug, but again HOCl was more potent than H2O2. When D-penicillamine was oxidised by exposure to H2O2 it could no longer serve as a scavenger of secreted oxidants from neutrophils. These data suggest that in vivo the preferential scavenging of HOCl may be important under pathological conditions where secreted myeloperoxidase may be functional.
=======================================================================
14.) Lack of induction of antinuclear antibodies by D-penicillamine in rheumatoid arthritis: a controlled study.
=======================================================================
SO - J Rheumatol 1986 Apr;13(2):308-12
AU - Weinstein A; Rothfield NF
PT - JOURNAL ARTICLE
AB - The induction of antinuclear antibodies (ANA) by D-penicillamine (DP) was studied in 148 patients with rheumatoid arthritis (RA) who had completed at least 30 weeks in a double blind trial which compared DP at doses 500 mg and 125 mg daily to placebo. There was no difference among the 3 groups in the frequency of a positive ANA at any titer or at a titer greater than 1:16 either pretreatment or at the end of the study. The conversion from a negative to a positive ANA occurred as frequently in the placebo treated controls with RA as in the DP treated RA patients. Antibodies to native DNA appeared in 3 DP treated patients, none of whom had symptoms of drug induced lupus or of DP toxicity.
=======================================================================
15.) Influence of food on the bioavailability of penicillamine.
=======================================================================
SO - J Rheumatol 1983 Feb;10(1):95-7
AU - Schuna A; Osman MA; Patel RB; Welling PG; Sundstrom WR
PT - JOURNAL ARTICLE
AB - The relative bioavailability of D-penicillamine was determined after single 500 mg oral doses of commercial tablets to healthy male volunteers under fasting and nonfasting conditions. In fasted individuals the mean maximum penicillamine level in plasma of 3.05 mcg/ml occurred at 3.8 h, and the area under the 0-12 h plasma curve was 14.7 mcg/h/ml. In nonfasted individuals the mean maximum penicillamine level of 1.52 mcg/ml occurred at 2.3 h, and the area under the 0-12 h plasma curve was reduced to 7.16 mcg/h/ml. Thus under these conditions food reduced systemic penicillamine availability by 1/2, but did not reduce the apparent absorption rate.
=======================================================================
16.) Treatment of generalized scleroderma with inhibitors of collagen synthesis.
=======================================================================
SO - Int J Dermatol 1982 Apr;21(3):159-61
AU - Asboe-Hansen G
PT - JOURNAL ARTICLE
AB - Clinical evaluation of scleroderma and the urinary excretion of collagen specific amino acids and uronic acid indicate disease activity and have become a guide for treatment. During an experimental search for inhibitors of the synthesis of collagen and ground substance glycosaminoglycans, a few connective-tissue active agents were selected for therapy of scleroderma: D-penicillamine, benzyl-penicillin-diethylamino-ethylester-hydroiodide, L-glutamine, hydralazine, glucocorticoids, dextro-thyroxine, L-dopa, diphenylhydantion, chlorpromazine, and (+) catechine. Treatment for several years was required to bring about an arrest of progression in 89% of the patients, a regression in three-quarters, and subtotal or total recovery in more than 40%. Indications of favorable prognosis are youth, short disease history, a high total dose of agent, and long duration of the treatment.
=======================================================================
17.) Role of disease-modifying antirheumatic drugs versus cytotoxic agents in the therapy of rheumatoid arthritis.
=======================================================================
SO - Am J Med 1988 Oct 14;85(4A):39-44
AU - Ward JR
AD - Division of Rheumatology, University of Utah School of Medicine, Salt Lake City 84132.
MJ - Anti-Inflammatory Agents [therapeutic use]; Antineoplastic Agents [therapeutic use]; Arthritis, Rheumatoid [drug therapy]
MN - Arthritis, Rheumatoid [physiopathology]; Clinical Trials; Immunosuppressive Agents [therapeutic use]
MT - Comparative Study; Human
PT - CLINICAL TRIAL; JOURNAL ARTICLE; REVIEW (34 references); REVIEW, TUTORIAL
AB - Disease-modifying antirheumatic drugs are used to modify or alter the rheumatoid arthritis disease process. Disease-modifying antirheumatic drugs do not demonstrate the characteristic analgesic, antipyretic, and anti-inflammatory actions of the nonsteroidal anti-inflammatory drugs, since weeks or months of treatment are required before clinical benefit is observed. Although they have not been proved to delay, prevent, or reverse articular damage, therapy with disease-modifying antirheumatic drugs, when successful, is associated with decreased pain and joint swelling and improved function. Disease-modifying antirheumatic drugs and cytotoxic agents should not be considered as routine treatment for patients with rheumatoid arthritis. Before disease-modifying antirheumatic drug therapy is implemented, an optimal basic program of physical therapy, rest, and nonsteroidal anti-inflammatory drugs should be implemented, and it must be documented that the patient still has sufficient disease to justify the costs, risks, and benefits of these treatments. Drugs that are approved by the Food and Drug Administration (FDA) are preferred over nonapproved drugs. Hydroxychloroquine, parenteral gold salts, oral gold, D-penicillamine, and the cytotoxic drug azathioprine are the FDA-approved disease-modifying antirheumatic drugs for use in rheumatoid arthritis. Many, not all, rheumatologists would employ hydroxychloroquine as the first-choice disease-modifying antirheumatic drug in patients who have early, mild, and nonerosive disease; treatment should be continued for six months before being abandoned for lack of efficacy, and appropriate ophthalmologic examination every four to six months is indicated. An alternative would be auranofin, whose efficacy approaches that of parenteral gold, yet may be safer. For patients who have severe active rheumatoid arthritis, especially with erosive changes, parenteral gold salts are usually a first choice. D-penicillamine is also effective in controlling the signs and symptoms of rheumatoid arthritis, but serious toxicity may occur. Azathioprine might be considered a competitor to D-penicillamine, although the FDA approval restricts its use to patients who have failed gold therapy. Two cytotoxic drugs that are not FDA approved are methotrexate and cyclophosphamide. Methotrexate can be very effective, but its side effects, particularly pulmonary and hepatic, must be carefully monitored. Cyclophosphamide is generally considered too toxic for use in patients with rheumatoid arthritis, although it may be helpful in patients with systemic rheumatoid vasculitis or patients who have failed all other therapies.(ABSTRACT TRUNCATED AT 400 WORDS).
=======================================================================
18.) Intermittent treatment with D-penicillamine is effective in lower doses and with fewer adverse effects in patients with rheumatoid arthritis.
=======================================================================
SO - J Rheumatol 1994 Sep;21(9):1637-41
AU - Hakoda M; Taniguchi A; Kamatani N; Akahoshi T; Kashiwazaki S
AD - Institute of Rheumatology, Tokyo Women's Medical College, Japan.
AB - OBJECTIVE. To determine whether intermittent rather than daily administration of D-penicillamine (D-Pen) would effectively reduce the incidence of adverse effects without significantly diminishing the clinical benefits. METHODS. We conducted an open prospective trial comparing daily and intermittent schedules. Among 76 Japanese patients with rheumatoid arthritis (RA), 37 underwent daily treatment with D-Pen while 39 were given D-Pen intermittently (every Monday, Wednesday, and Friday). RESULTS. The mean D-Pen dose was 166.8 and 99.4 mg/day for daily and intermittent groups, respectively, the difference being highly significant (p 0.0001, Mann-Whitney's U test). The incidence of adverse effects was significantly lower in the intermittent group. Both schedules significantly reduced the activity of RA, as evaluated by clinical and laboratory variables. No significant differences were observed in the degree of improvement between the 2 schedules. CONCLUSION. Intermittent therapy with D-Pen is an effective treatment for patients with RA and its higher degree of flexibility can lead to maximum efficacy for management of patients with RA.
=======================================================================
19.) A western blot approach to detection of human plasma protein conjugates derived from D-penicillamine.
=======================================================================
SO - Ann Rheum Dis 1994 Apr;53(4):256-60
AU - Laycock CA; Phelan MJ; Bucknall RC; Coleman JW
AD - Department of Pharmacology and Therapeutics, University of Liverpool, United Kingdom.
PT - JOURNAL ARTICLE
AB - OBJECTIVES--To develop and apply an immunochemical approach to the study of drug-plasma protein conjugates derived from the anti-arthritic drug D-penicillamine (DP). METHODS--An antiserum with specificity for protein-conjugated DP was raised in a rabbit. Plasma samples from patients receiving DP or from incubations of isolated normal plasma with DP were analysed for DP-derived conjugates by Western blotting using the anti-drug antibody. RESULTS--A single DP-positive protein band was detected in plasma samples from 15/16 patients with rheumatoid arthritis receiving DP but in none of 20 patients of similar disease status who had not taken DP. The positive band appeared in patients' plasma during the course of treatment with DP. It was seen under nonreducing but not reducing conditions indicating that the drug is disulphide linked to the protein. The drug-modified protein migrated to a position intermediate between the trailing edge of albumin and the leading edge of transferrin (both non-reduced) suggesting a molecular weight of between 66 and 77 kDa. Incubations of normal human plasma, but not purified albumin or transferrin, with low concentrations of DP generated the same distinct band plus several less intense DP-positive bands. CONCLUSIONS--Drug-plasma protein conjugates derived from DP in vivo and in vitro can be detected immunochemically by the Western blot method. Theories of DP immunotoxicity have implicated antigenicity of the drug, but this is the first immunochemical demonstration of a potential DP-derived antigen in human plasma. The method we describe may have application to studies of the relationship between DP antigenicity and toxicity.
=======================================================================
20.) Disease-modifying antirheumatic drugs, including methotrexate, sulfasalazine, gold, antimalarials, and D-penicillamine.
=======================================================================
SO - Curr Opin Rheumatol 1993 May;5(3):276-81
AU - Conaghan PG; Brooks P
AD - St. Vincent's Hospital, Sydney, Australia.
PT - JOURNAL ARTICLE; REVIEW (44 references); REVIEW, TUTORIAL
AB - Rheumatoid arthritis is a progressive inflammatory disease with evidence of early cartilage damage. Consequently, there is a trend toward earlier use of disease-modifying antirheumatic drugs. This review focuses on recent data on methotrexate, sulfasalazine, gold, hydroxychloroquine, D-penicillamine, and combination therapy. Methotrexate is focused on most, reflecting the increasing popularity of this agent; studies continue to show its good clinical efficacy. Combination therapy studies have been disappointing and are still marred by short duration and low power. Sulfasalazine has a fairly rapid onset of clinical effects and modulates immune function. Gold is still the subject of new studies, with evidence that clinical experience leads to improved patient efficacy. The beneficial effect of hydroxychloroquine in mild rheumatoid arthritis has been confirmed, and information on D-penicillamine suggests an effect on oxygen radical scavenging. Clinical studies are still required to ascertain the relative efficacy of these drugs, and potential long-term adverse effects remain a source of concern.
=======================================================================
21.) Frequent induction of insulin autoantibodies by D-penicillamine in patients with rheumatoid arthritis.
=======================================================================
SO - J Rheumatol 1992 Oct;19(10):1527-30
AU - Vardi P; Brik R; Barzilai D; Lorber M; Scharf Y
AD - Department of Rheumatology, Rambam Medical Center, Technion School of Medicine, Haifa, Israel.
PT - JOURNAL ARTICLE
AB - D-Penicillamine is a drug known to induce various immunological abnormalities. We used a competitive radiobinding assay, to evaluate the presence of insulin autoantibodies in 42 patients with rheumatoid arthritis (RA), under various treatment modalities. In 11/42 (26.2%) patients, the levels of insulin autoantibodies (range 59-1970 nU/ml) exceeded our upper limit of normal range (50 nU/ml). Nine of these 11 (81.8%) insulin autoantibodies positive patients had been treated with D-penicillamine. Out of 21 D-penicillamine treated patients, 9 (42.9%) were insulin autoantibodies positive (range 80 to 1970 nU/ml). An inverse correlation was found between the concentration of insulin autoantibodies and the time interval since the last drug administration, R = -0.58 (p 0.05). No correlation was found between the autoantibodies levels and age, or duration of D-penicillamine treatment. In summary, elevated concentration of serum insulin autoantibodies are most probably induced by D-penicillamine therapy in patients with RA and tend to decrease after the drug withdrawal.
=======================================================================
22.) The fasciitis-morphea complex in children.
=======================================================================
SO - Am J Dis Child 1992 Jun;146(6):733-6
AU - Miller JJ 3d
AD - Lucille Salter Packard Children's Hospital, Stanford, Palo Alto, CA 94304.
PT - JOURNAL ARTICLE
AB - OBJECTIVE--To describe the clinical presentation and treatment of the combined syndrome of fasciitis and morphea in children. SETTING--The rheumatic disease clinic of a university children's hospital. PATIENTS--Six children who were referred for signs or symptoms of fasciitis were observed. SELECTION PROCEDURES--All children who were referred for fasciitis were included in the study. INTERVENTIONS--Therapy included prednisone initially and subsequent long-term treatment with penicillamine and alternate-day doses of prednisone. MEASUREMENTS AND RESULTS--Symptoms of morphea appeared 1 year before to 4 years after the first signs of fasciitis were observed. The fasciitis was characterized by usually symmetrical, centrifugal swelling, pain, and contracture formation. The morphea and fasciitis did not appear in the same areas of the body. CONCLUSIONS--The fasciitis and morphea are clearly linked manifestations. The fasciitis, but not the morphea, appears to respond to treatment with steroids. Treatment with penicillamine may ameliorate the sclerosis of the morphea but does not stop new lesions from appearing.
=======================================================================
23.) The interactions of penicillamine with copper in vivo and the effect on hepatic metallothionein levels and copper/zinc distribution: the implications for Wilson's disease and arthritis therapy.
=======================================================================
SO - J Lab Clin Med 1992 Jun;119(6):744-50
AU - McQuaid A; Lamand M; Mason J
AD - Biochemistry Department, Trinity College, Dublin, Ireland.
PT - JOURNAL ARTICLE
AB - D-penicillamine does not remove copper from metallothionein, but it has been suggested that it may increase hepatic metallothionein levels. D-penicillamine was shown to increase rat hepatic metallothionein levels; however, the effect was dependent on an interaction with copper. The drug accelerated the excretion of exogenous copper but increased the amount retained on metallothionein. This interaction of penicillamine and copper also provoked changes in the distribution of zinc and in particular an increase in the heat-stable cytosol zinc fraction. In contrast, thiomolybdates were much more effective in eliminating exogenous copper and even removed copper that was already bound to metallothionein; thus, the copper level in the heat-stable cytosol fraction decreased. The observations support the view that patients with Wilson's disease may not be truly "decoppered" but that treatment with d-penicillamine is effective because the accumulated copper in the liver is bound in a nontoxic form by the increased metallothionein. The results explain why cessation of treatment is dangerous. The results may also partially explain the effectiveness of D-penicillamine copper chelates as antiinflammatory drugs.
=======================================================================
24.) Comparison of cyclosporine and D-penicillamine for rheumatoid arthritis: a randomized, double blind, multicenter study.
=======================================================================
SO - J Rheumatol 1991 Jun;18(6):815-20
AU - van Rijthoven AW; Dijkmans BA; The HS; Meijers KA; Montnor-Beckers ZL; Moolenburgh JD; Boers M; Cats A
AD - Department of Rheumatology, Deaconess Hospital, Eindhoven, The Netherlands.
PT - CLINICAL TRIAL; JOURNAL ARTICLE; MULTICENTER STUDY; RANDOMIZED CONTROLLED TRIAL
AB - Ninety-two patients with active rheumatoid arthritis (RA) were entered in a randomized double blind study of 24 weeks comparing cyclosporine (initial daily dose 5 mg/kg) with D-penicillamine (initial daily dose 250 mg). The groups were well balanced in baseline characteristics. In the cyclosporine group, 10 patients stopped prematurely, one because of inefficacy. In the D-penicillamine group, 9 patients stopped prematurely, 3 because of inefficacy. The 2 antirheumatic drugs were equally effective in reducing disease activity, except for a significant (p = 0.005) decrease in erythrocyte sedimentation rate with D-penicillamine treatment. We conclude that under the conditions of this trial, cyclosporine can serve as an alternative to D-penicillamine for the treatment of patients with RA.
=======================================================================
25.) D-penicillamine in the treatment of localized scleroderma [see coms]
=======================================================================
CM - Comment in: Arch Dermatol 1990 May; 126(5):661-4
SO - Arch Dermatol 1990 May;126(5):609-12
AU - Falanga V; Medsger TA Jr
AD - Department of Dermatology and Cutaneous Surgery, University of Miami, School of Medicine, FL 33101.
PT - JOURNAL ARTICLE
AB - Localized scleroderma has no recognized internal organ involvement but may be disfiguring and disabling when the cutaneous lesions are extensive or affect children. There is no accepted or proven treatment for localized scleroderma. Case reports of 11 patients with severe, extensive localized scleroderma who were treated with D-penicillamine are summarized in this article. This drug was judged to have a favorable effect on the disease course in 7 (64%) of 11 patients. Improvement began within 3 to 6 months and consisted of cessation of active cutaneous lesions in all 7 patients, skin softening in 5, and more normal growth of the affected limb in 2 of 3 children. Joint stiffness and contractures also improved. The dose of D-penicillamine associated with a favorable response was as low as 2 to 5 mg/kg per day given over a period ranging from 15 to 53 months. D-Penicillamine caused nephrotic syndrome in 1 patient and milder reversible proteinuria in 3 other patients; none developed renal insufficiency. These data suggest that D-penicillamine may be effective in severe cases of localized scleroderma.
=======================================================================
26.) Inhibition of human endothelial cell proliferation in vitro and neovascularization in vivo by D-penicillamine.
=======================================================================
SO - J Clin Invest 1989 Jan;83(1):158-67
AU - Matsubara T; Saura R; Hirohata K; Ziff M
AD - Department of Orthopedic Surgery, Kobe University School of Medicine, Japan.
PT - JOURNAL ARTICLE
AB - To investigate the effects of D-penicillamine (D-Pen) on angiogenesis, we have studied the effects of this drug on in vitro proliferation of human endothelial cells (EC) and in vivo corneal neovascularization. D-Pen, in the presence of copper sulfate, suppressed tritiated thymidine ([3H]TdR) incorporation into EC in a dose-dependent manner. Significant inhibition was observed with D-Pen concentrations attainable in the serum and tissues of treated patients. Neither D-Pen nor copper ion alone significantly affected [3H]TdR incorporation into EC. The inhibition by D-Pen and copper was blocked by catalase (CAT) or horseradish peroxidase but not by boiled CAT or SOD. When rabbits were daily injected intravenously with D-Pen at the per kilogram dosage administered to rheumatoid patients, neovascularization as quantitated by the proliferation of corneal new blood vessels was significantly inhibited. These results suggest that hydrogen peroxide generated by D-Pen and copper exerts a pronounced antiangiogenic effect through inhibition of EC proliferation. It is, therefore, considered that D-Pen may suppress rheumatoid synovitis by reducing the number of small blood vessels available for the emigration of chronic inflammatory cells, and the proliferation of the synovial tissue.
=======================================================================
27.) [Pemphigus herpetiformis following D-penicillamine in a patient with HLA B8]
=======================================================================
TT - [Pemphigus herpetiformis nach D-Penicillamin bei einem Patienten mit HLA B8.]
SO - Hautarzt 1988 Sep;39(9):587-8
AU - Weltfriend S; Ingber A; David M; Sandbank M
MC - English Abstract
PT - JOURNAL ARTICLE
AB - A 57-year-old patient with rheumatoid arthritis, who was being treated with D-penicillamine, developed a bullous eruption 2 months after the onset of treatment. On the basis of the clinical, histological and immunofluorescent findings, the diagnosis of herpetiform pemphigus was made. The eruptions disappeared within 2 weeks after the discontinuation of D-penicillamine treatment. During the 16-month follow-up period there was no recurrence. It is noteworthy that a patch test with potassium iodide was positive and that testing for HLA antigens revealed HLA B8, A1, A2, and BW39.
=======================================================================
28.) Progressive systemic sclerosis complicated with immune thrombocytopenia
during D-penicillamine therapy.
=======================================================================
Author
Natsuda H; Matsui Y; Sakauchi M; Kato S; Takemura H; Suzuki H; Kono I;
Yamane K; Kashiwagi H
Address
Department of Internal Medicine, University of Tsukuba, Japan.
Source
Intern Med, 31(2):244-5 1992 Feb
Abstract
Immune thrombocytopenia is a rare complication of progressive systemic
sclerosis (PSS). A 47-year-old female with PSS treated with D-penicillamine
developed immune thrombocytopenia, which promptly responded to prednisolone
and withdrawal of D-penicillamine. Platelet-associated IgG was elevated and
the bone marrow megakaryocyte count was normal. There was an inverse
relationship between the level of platelet-associated IgG and the platelet
count. A lymphocyte stimulation test sensitized by D-penicillamine was
positive. The present case suggests that immune thrombocytopenia may be
regarded as one of the D-penicillamine-related immune abnormalities. To our
knowledge, its association with PSS has never been reported.
=======================================================================
29.) Lupus erythematosus-like eruption due to D-penicillamine in progressive
systemic sclerosis.
=======================================================================
Author
Tsankov NK; Lazarova AZ; Vasileva SG; Obreshkova EV
Address
Department of Medicine, Academy of Medicine, Sofia, Bulgaria.
Source
Int J Dermatol, 29(8):571-4 1990 Oct
Abstract
A 58-year-old woman with progressive systemic sclerosis developed a
butterfly erythematous eruption, hemorrhagic maculae, crusting, and
erosions on the face 15 days following administration of D-penicillamine.
This appeared to be a lupus erythematosus-like eruption due to an adverse
drug reaction to D-penicillamine because of the rapid recovery from the
general symptoms, the total involution of skin lesions with topical
treatment only, the transient demonstration of autoantibodies and their
fast negativation, negative ANA, and the histologic features of lupus
erythematosus combined with marked exudative changes.
=======================================================================
30.) Bronchiolitis obliterans in a patient with localized scleroderma treated
with D-penicillamine.
=======================================================================
Author
Boehler A; Vogt P; Speich R; Weder W; Russi EW
Address
Dept of Internal Medicine, University Hospital, Zuerich, Switzerland.
Source
Eur Respir J, 9(6):1317-9 1996 Jun
Abstract
D-penicillamine-associated bronchiolitis obliterans (BO) is a rare but
well-known pulmonary complication in patients with rheumatoid arthritis or
progressive systemic sclerosis. It has been assumed that in most, if not
all cases, BO is a complication of the underlying disease rather than a
side-effect of treatment. We report the case of a 46 year old man with
scleroderma localized to his lower legs (morphea), who received a daily
dose of 750 mg D-penicillamine. During the treatment of 1 yr duration, he
developed progressive shortness of breath due to a worsening obstructive
ventilatory defect suggesting BO, which was confirmed by surgical lung
biopsy (constrictive BO). Bronchial obstruction progressed over the next 5
yrs and did not respond to corticosteroids. The patient finally underwent a
successful single left lung transplantation. The histological features of
constrictive BO were confirmed in the explanted lung. This observation
suggests that D-penicillamine may induce bronchiolitis obliterans in the
absence of a systemic connective tissue disease.
=======================================================================
31.) [Extracapillary glomerulonephritis secondary to D-penicillamine. Apropos of
1 case and review of the literature]
=======================================================================
Author
Marchand-Courville S; Dhib M; Fillastre JP; Godin M
Address
Service de n´ephrologie, CHU de Rouen.
Source
Nephrologie, 19(1):25-32 1998
Abstract
Extracapillary glomerulonephritis was diagnosed in a 51-year-old woman
after 11 months of D-Penicillamine treatment given for systemic sclerosis.
Antineutrophil cytoplasmic antibodies specific for myeloperoxydase were
detected. Penicillamine was stopped and the patient was treated with plasma
exchanges, cyclophosphamide and corticosteroids. The renal function
progressively deteriorated leading to end stage requiring dialysis.
Previous reports of extracapillary glomerulonephritis after D-penicillamine
are analysed. Several cases with alveolar haemorrhage are consistent with
the diagnosis of microscopic polyangiitis.
=======================================================================
32.) Unilateral ptosis as an initial manifestation of D-penicillamine induced
myasthenia gravis.
=======================================================================
Author
Raynauld JP; Lee YS; Kornfeld P; Fries JF
Address
Division of Immunology and Rheumatology, Stanford University Medical
Center, CA.
Source
J Rheumatol, 20(9):1592-3 1993 Sep
Abstract
We describe 2 patients presenting with isolated unilateral ptosis without
other signs of cranial or peripheral nerve involvement or sympathetic
denervation. Both patients (one case of progressive systemic sclerosis and
one of rheumatoid arthritis) were currently taking D-penicillamine. In
these cases, the ptosis was reversed a few minutes after a Tensilon test,
hallmark of myasthenia gravis. Antibodies to acetylcholine receptors were
present. Myasthenia gravis should be suspected with ptosis without other
cranial nerve involvement or miosis, even if the ptosis is unilateral.
Thus, unilateral ptosis can be the first manifestation of a toxic side
reaction to D-penicillamine.
=======================================================================
33.) C1 inhibitor deficiency in a patient with rheumatoid arthritis--increased
risk of adverse effects of penicillamine?
=======================================================================
Author
Sturfelt G; Rydgren L; Truedsson L; Alm P; Sj¨oholm AG
Address
Department of Rheumatology, Lund University Hospital, Sweden.
Source
J Rheumatol, 23(2):378-81 1996 Feb
Abstract
We report the coincidence of hereditary angioedema and rheumatoid arthritis
in a male patient and in his father. During treatment with D-penicillamine
the patient developed a transient lupus-like disorder with
glomerulonephritis that resolved when D-penicillamine was discontinued. He
later was diagnosed with malignant lymphoma. Impaired classical complement
pathway function could have contributed to development of the drug reaction.
=======================================================================
34.) D-penicillamine- and quinidine-induced antinuclear antibodies in A.SW
(H-2s) mice: similarities with autoantibodies in spontaneous and heavy
metal-induced autoimmunity.
=======================================================================
Author
Monestier M; Novick KE; Losman MJ
Address
Garden State Cancer Center, Newark, NJ.
Source
Eur J Immunol, 24(3):723-30 1994 Mar
Abstract
Ten percent of human lupus syndromes occur in patients as a result of
treatment with certain medications. H-2s mice can produce autoantibodies
following treatment with various drugs or heavy metals and they are a
potential animal model of drug-induced lupus. We have examined nine
anti-chromatin monoclonal antibodies (mAb) from A.SW mice that had been
treated with either D-penicillamine or quinidine, two lupus-inducing drugs
in humans. These mAb are specific either for DNA or histone-DNA complexes
corresponding to nucleo-specific either for DNA or histone-DNA complexes
corresponding to nucleosomes or subnucleosome particles. Only one mAb
reacts with an unknown chromatin antigen. The V region sequences of six of
these mAb were studied and are notable by several features. As previously
observed in spontaneous autoantibodies to DNA or histone-DNA complexes,
arginine or asparagine residues are found at critical locations throughout
the V regions. Many of these residues, potentially important for binding to
DNA or DNA-histone complexes, result either from somatic mutations or
atypical VH-D-JH rearrangements. Another significant characteristic is that
the VH genes of several D-penicillamine- or quinidine-induced mAb are most
similar to those of anti-nucleolar mAb obtained from mercury-injected A.SW
mice. The implications of these findings for the pathogenesis of
spontaneous or induced autoimmunity are discussed.
=======================================================================
35.) Combination therapy for rheumatoid arthritis and drug-induced systemic
lupus erythematosus.
=======================================================================
Author
Borg AA; Davis MJ; Dawes PT; Shadforth MF
Address
Dept. of Rheumatology, Freeman Hospital, Newcastle-upon-Tyne.
Source
Clin Rheumatol, 13(3):522-4 1994 Sep
Abstract
Development of drug-induced systemic lupus erythematosus (SLE) is an
uncommon complication of the use of D-penicillamine and sulphasalazine. We
report two cases of patients with rheumatoid arthritis (RA) who developed
symptoms and signs of SLE and suggest that increasing use of these two
agents as combination therapy in RA may cause an additive risk to the
occurrence of this complication.
=======================================================================
36.) Chronic modulation of the autoimmune response following parvovirus B19
infection.
=======================================================================
Author
Vigeant P; M´enard HA; Boire G
Address
Rheumatic Diseases Unit, Faculty of Medicine, Universit´e de Sherbrooke,
Centre hospitalier universitaire de Sherbrooke, PQ, Canada.
Source
J Rheumatol, 21(6):1165-7 1994 Jun
Abstract
We describe 2 patients with prolonged autoimmune alterations following
parvovirus B19 infection. B19 induced aplastic crises were the revealing
manifestations of asymptomatic hemolytic conditions in the 2 patients: a
Coombs' positive hemolytic anemia induced by D-penicillamine in the first
and congenital spherocytosis in the second. Both patients had transient
clinical and serological manifestations highly suggestive of systemic lupus
erythematosus. In addition, prolonged clinical and serological remission of
rheumatoid arthritis was observed in the first patient, while arthralgias,
FANA, and anti-Ro antibodies persisted in the second, previously healthy
patient. Our data suggest that parvovirus B19 infection may lead to chronic
modulation of the autoimmune response in predisposed individuals.
=======================================================================
37.) Massive breast enlargement in a patient receiving D-penicillamine for systemic sclerosis.
=======================================================================
SO - J Rheumatol 1985 Oct;12(5):990-1
AU - Kahl LE; Medsger TA Jr; Klein I
PT - JOURNAL ARTICLE
AB - Breast enlargement is an unusual complication of D-penicillamine therapy for rheumatoid arthritis. We observed breast enlargement and hyperprolactinemia in a woman receiving D-penicillamine for systemic sclerosis. Treatment with danazol was associated with a reduction in both the breast size and the prolactin level.
=======================================================================
38.) Progressive systemic sclerosis complicated with immune thrombocytopenia during D-penicillamine therapy.
=======================================================================
SO - Intern Med 1992 Feb;31(2):244-5
AU - Natsuda H; Matsui Y; Sakauchi M; Kato S; Takemura H; Suzuki H; Kono I; Yamane K; Kashiwagi H
PT - JOURNAL ARTICLE
AB - Immune thrombocytopenia is a rare complication of progressive systemic sclerosis (PSS). A 47-year-old female with PSS treated with D-penicillamine developed immune thrombocytopenia, which promptly responded to prednisolone and withdrawal of D-penicillamine. Platelet-associated IgG was elevated and the bone marrow megakaryocyte count was normal. There was an inverse relationship between the level of platelet-associated IgG and the platelet count. A lymphocyte stimulation test sensitized by D-penicillamine was positive. The present case suggests that immune thrombocytopenia may be regarded as one of the D-penicillamine-related immune abnormalities. To our knowledge, its association with PSS has never been reported.
=======================================================================
39.) Systemic sclerosis-like lesions during long-term penicillamine therapy for Wilson's disease.
=======================================================================
SO - Br J Dermatol 1987 Jan;116(1):95-100
AU - Miyagawa S; Yoshioka A; Hatoko M; Okuchi T; Sakamoto K
MJ - Hepatolenticular Degeneration [drug therapy]; Penicillamine [adverse effects]; Scleroderma, Systemic [chemically induced]
MN - Adolescence; Hepatolenticular Degeneration [pathology]; Penicillamine [therapeutic use]; Scleroderma, Systemic [pathology]
MT - Case Report; Human; Male; Support, Non-U.S. Gov't
PT - JOURNAL ARTICLE
AB - Systemic sclerosis-like lesions developed in a 14-year-old boy with Wilson's disease who had been treated with D-penicillamine for 11 years. Clinical and laboratory manifestations included proximal scleroderma, pulmonary restrictive defects, positive antinuclear antibodies, and the deposition of C3 at the dermal-epidermal junction of the lesional skin. This is the first case reported in which long-term administration of penicillamine was followed by the development of systemic sclerosis-like lesions.
=======================================================================
40.) The toxicity of D-penicillamine in systemic sclerosis.
=======================================================================
SO - Ann Intern Med 1986 May;104(5):699-705
AU - Steen VD; Blair S; Medsger TA Jr
PT - JOURNAL ARTICLE
AB - We studied D-penicillamine toxicity in 259 patients with systemic sclerosis treated since 1972. The average daily dose of 635 mg was given for a mean of 1.8 years. Of patients with systemic sclerosis, 47% has side effects from D-penicillamine treatment, similar to the 56% of 807 patients with rheumatoid arthritis in seven separate series. Individual manifestations of toxicity included rash, proteinuria, gastrointestinal symptoms, dysgeusia, oral ulcers, thrombocytopenia, and neutropenia. Four episodes each of myasthenia gravis and pemphigus occurred in our patients; both were reported rarely in patients with rheumatoid arthritis. Adverse effects occurred more frequently after rapid increases in dosage. Treatment had to be discontinued due to toxicity in 29% of patients with systemic sclerosis and in 33% of those with rheumatoid arthritis. Although toxic, with a high frequency of adverse effects, D-penicillamine can be used safely in the treatment of systemic sclerosis. Pemphigus and myasthenia gravis may occur more frequently with therapy for systemic sclerosis than with that for rheumatoid arthritis.
=======================================================================
41.) D-penicillamine induced crescentic glomerulonephritis: report and review of the literature.
=======================================================================
SO - J Rheumatol 1987 Oct;14(5):1036-41
AU - Devogelaer JP; Pirson Y; Vandenbroucke JM; Cosyns JP; Brichard S; Nagant de Deuxchaisnes C
AD - Rheumatology Unit, Saint-Luc University Hospital, Louvain University in Brussels, Belgium.
PT - JOURNAL ARTICLE
AB - A 56-year-old woman with scleroderma developed rapidly progressive glomerulonephritis with epithelial crescents associated with hemoptysis after 27 months of D-penicillamine therapy and a cumulative dose of 1,200 g. Renal failure necessitated 5 hemodialysis sessions. D-penicillamine was withdrawn and glucocorticoids combined with azathioprine were given with good recovery of renal function. The 9 other reported cases of D-penicillamine induced rapidly progressive glomerulonephritis have been reviewed. This syndrome is potentially life-threatening: the 5 untreated patients died, whereas 5 patients given immunosuppressive therapy are alive.
=======================================================================
42.) Antibodies to cellular antigens in Greek patients with autoimmune rheumatic diseases: anti-Ro(SSA) antibody a possible marker of penicillamine-D intolerance.
=======================================================================
SO - Ann Rheum Dis 1984 Apr;43(2):285-7
AU - Moutsopoulos HM; Giotaki H; Maddison PJ; Mavridis AC; Drosos AA; Skopouli FN
PT - JOURNAL ARTICLE
AB - One hundred and twenty-four sera from Greek patients with autoimmune rheumatic diseases (29 with systemic lupus erythematosus (SLE), 24 with scleroderma, 11 with primary Sjogren's syndrome (SS), and 60 with rheumatoid arthritis (RA) were tested for antibodies to nRNP, Sm, Scl-70, Ro(SSA), and La(SSB) cellular antigens. The incidence of these antibodies in the different groups of patients examined, did not differ overall from that described previously. It was noted, however, that antibodies to Sm were very infrequently found in Greek patients with SLE and anti-Ro positive patients with SLE did not have the clinical manifestations described by other workers. Finally, it was found that anti-Ro positive patients with RA experienced a high frequency of side effects from penicillamine-D. The significance of these findings is discussed.
=======================================================================
43.) HLA system and side effects of gold salts and D-penicillamine treatment of rheumatoid arthritis.
=======================================================================
SO - Ann Rheum Dis 1982 Dec;41(6):599-601
AU - Bardin T; Dryll A; Debeyre N; Ryckewaert A; Legrand L; Marcelli A; Dausset J
MT - Female; Human; Male
PT - JOURNAL ARTICLE
AB - Among 67 patients with rheumatoid arthritis treated with gold salts (aurothiopropanol sulphonate) a significant correlation (p less than 10(-2)) was noted between gold toxic reactions, whatever their type, and the HLA antigens A1, B8, Cw7, and DR3. Forty-two patients were genotyped, and a correlation was observed between gold side effects and the haplotype A1 Cw7 B8 DR3 (p less than 10(-2), RR = 8.0). In addition 3 out of 4 cases of renal intolerance to D-penicillamine were observed in patients possessing the Cw7 B8 DR3 haplotype.
=======================================================================
44.) In vitro inhibition of myelopoiesis by gold salts and D-penicillamine.
=======================================================================
SO - J Rheumatol 1985 Oct;12(5):892-6
AU - Hamilton JA; Williams N
PT - JOURNAL ARTICLE
AB - Leukopenia is one of the more serious side effects of gold and D-penicillamine therapy. We report that gold salts (gold thiomalate, gold chloride and gold thioglucose) and D-penicillamine suppressed the in vitro development of colonies of myeloid cells (macrophage, granulocyte and megakaryocyte) from progenitor cells in murine bone marrow; these drugs were also effective in inhibiting the development of macrophage and granulocytic colonies in human bone marrow. The drugs generally required concentrations of 10(-5) M in the murine system, whereas they were active at 10(-7)-10(-8) for the human progenitor cells. The disease suppressive activity of gold salts and D-penicillamine could result in part from the reduction of cell numbers in arthritic lesions; our findings would provide a mechanism for this possibility.
=======================================================================
45.) Autoimmune associations in primary biliary cirrhosis.
=======================================================================
SO - Mayo Clin Proc 1982 Jun;57(6):365-70
AU - Culp KS; Fleming CR; Duffy J; Baldus WP; Dickson ER
PT - JOURNAL ARTICLE
AB - The prevalence of autoimmune associations was determined in 113 patients with primary biliary cirrhosis who participated in a therapeutic trial of D-penicillamine. Eighty-four percent of the patients had at least one associated autoimmune disease and 41% had two or more such diseases in addition to primary biliary cirrhosis. Keratoconjunctivitis sicca, which was present in 66% of the patients, was the most commonly associated autoimmune disease. In most of the patients the autoimmune disease was recognized after the diagnosis of primary biliary cirrhosis had been made. The prevalence of autoantibodies in selected subgroups of patients ranged from 70% (rheumatoid factor) to 22% (anti-native DNA). Polyclonal elevation of serum immunoglobulins was a consistent finding, and frequently all three major isotypes were simultaneously increased. Only IgA elevations correlated with histologic progression of primary biliary cirrhosis. The prominent involvement of epithelial tissues in the autoimmune disease of primary biliary cirrhosis suggests that an autoimmune syndrome affecting the secretory immune system is associated with the pathogenesis of primary biliary cirrhosis and the coexisting autoimmune diseases.
=======================================================================
46.) Sequential histopathologic alterations in Indian childhood cirrhosis treated with d-penicillamine [see comments]
=======================================================================
CM - Comment in: Hum Pathol 1991 Jul; 22(7):633
SO - Hum Pathol 1991 Jul;22(7):653-8
AU - Bhusnurmath SR; Walia BN; Singh S; Parkash D; Radotra BD; Nath R
PT - JOURNAL ARTICLE
AB - Eight children who satisfied all the diagnostic criteria of classic Indian childhood cirrhosis were treated with d-penicillamine. Clinical recovery in a 3- to 12-month period was accompanied histopathologically by accentuation of micronodules with regression of hepatocytic degenerative changes, Mallory's hyaline, pericellular fibrosis, lobular inflammation, and disappearance of hepatocytic copper staining protein. The nodules in the posttreatment biopsies were so small as to be categorized as "micronodular cirrhosis." In one case clinical recovery was associated with an almost normal liver histology after passing through a micronodular phase. This report is the first documentation of the histologic sequence of changes in Indian childhood cirrhosis on d-penicillamine treatment.
=======================================================================
47.) Pure red cell aplasia caused by D-penicillamine treatment of rheumatoid arthritis.
=======================================================================
SO - Ann Rheum Dis 1991 Apr;50(4):255-6
AU - Tishler M; Kahn Y; Yaron M
AD - Department of Rheumatology, Tel Aviv Medical Center, Ichilov Hospital, Israel.
MT - Case Report; Female; Human
PT - JOURNAL ARTICLE
AB - A 40 year old woman with rheumatoid arthritis developed pure red cell aplasia after treatment with D-penicillamine 500 mg/day. D-Penicillamine was stopped and short term treatment with steroids resulted in complete recovery of bone marrow.
=======================================================================
48.) A case of bullous pemphigoid induced by tiobutarit (D-penicillamine analogue).
=======================================================================
SO - J Dermatol 1989 Aug;16(4):308-11
AU - Yamaguchi R; Oryu F; Hidano A
PT - JOURNAL ARTICLE
AB - A 61-year-old woman with severe rheumatoid arthritis developed bullous lesions on the trunk, conjunctiva, and oral mucosa during treatment with tiobutarit, a new antirheumatic drug analogus to D-penicillamine. Histology showed a subepidermal blister, and circulating antibodies to the basement membrane zone were detected. The bullous lesions improved rapidly after discontinuation of tiobutarit. However, 3 years later, she was re-exposed to tiobutarit and the bullous lesions recurred within a week. This is the first confirmed case of tiobutarit-induced BP.
=======================================================================
49.) Fatal aplastic anaemia and liver toxicity caused by D-penicillamine treatment of rheumatoid arthritis.
=======================================================================
SO - Ann Rheum Dis 1989 Jul;48(7):609-10
AU - Fishel B; Tishler M; Caspi D; Yaron M
AD - Department of Rheumatology, Ichilov Hospital, Tel Aviv Medical Center, Israel.
PT - JOURNAL ARTICLE
AB - A 65 year old woman with rheumatoid arthritis developed marrow aplasia and jaundice owing to D-penicillamine treatment. Recovery of bone marrow was ineffective, and the patient finally died despite intensive therapeutic measures. The rare coexistence of myelotoxicity and hepatotoxicity is presented and discussed.
=======================================================================
50.) D-penicillamine-induced elastosis perforans serpiginosa in a child with juvenile rheumatoid arthritis. Report of a case and review of the literature.
=======================================================================
SO - J Am Acad Dermatol 1989 May;20(5 Pt 2):979-88
AU - Sahn EE; Maize JC; Garen PD; Mullins SC; Silver RM
AD - Department of Dermatology, Medical University of South Carolina, Charleston 29425-2215.
PT - JOURNAL ARTICLE; REVIEW (18 references); REVIEW OF REPORTED CASES
AB - Elastosis perforans serpiginosa is a rare complication of D-penicillamine therapy. It has been reported to occur in Wilson's disease and cystinuria, usually after many years of high-dose therapy. We report a case of D-penicillamine-induced elastosis perforans serpiginosa with unique clinical features occurring in a 10-year-old child with juvenile rheumatoid arthritis who received only 71 gm of the drug over 9 months. The case is also unusual because of the short course and low cumulative dose of drug received and because of the calcification of elastic fibers. The calcification of elastic fibers suggests that this case may represent an unusual variant of elastosis perforans serpiginosa or an overlap with pseudoxanthoma elasticum. All reported cases of D-penicillamine-induced elastosis perforans serpiginosa are reviewed, and histopathologic and electron microscopic findings are presented. The theoretic mechanisms of action of D-penicillamine on elastic tissue synthesis and morphology are discussed.
=======================================================================
51.) Nodular scleroderma in systemic sclerosis under D-penicillamine therapy.
=======================================================================
Author
Sasaki T; Denpo K; Ono H; Nakajima H
Address
Department of Dermatology, Yokohama City University School of Medicine,
Japan.
Source
J Dermatol, 19(12):968-71 1992 Dec
Abstract
A case of systemic sclerosis (SS) which developed keloidal lesions (nodular
scleroderma) on the chest during D-penicillamine (DPC) therapy is reported.
The 36-year-old woman showed rapidly progressing skin sclerosis with lung
and esophageal involvement, and DPC was started at the age of 38. Skin
sclerosis as a whole had improved to some extent, when keloidal nodules
developed on the upper chest at the age of 44. Since there were no other
findings suggestive of adverse reactions caused by DPC, we speculate that
activation of the fibroblasts in these lesions occurred despite the
suppressive effect of DPC.
=======================================================================
52.) D-PENICILLAMINE, the product
=======================================================================
CATEGORIES, BRAND NAMES, FORMULARIES & COST OF THERAPY
CATEGORIES: Antagonists and Antidotes; Antiarthritics; Arthritis; Chelating Agents; Cystinuria; FDA Approval Pre 1982; Heavy Metal Antagonists; Renal Drugs; Top 800 Drugs; Wilson's Disease
BRAND NAMES: Atamir*; Cuprimine; Cupripen*; Depen; Distamine*; Kelatin*; Mercaptyl*; Metalcaptase*; Pendramine*; Perdolat*; Sufortan*; Trolovol*
* Foreign brand name outside U.S.
FORMULARIES: Aetna; BC/BS; PCS; WHO
DESCRIPTION
************************************************************************
* *
* Physicians planning to use penicillamine should thoroughly *
* familiarize themselves with its toxicity, special dosage *
* considerations, and therapeutic benefits. Penicillamine should *
* never be used casually. Each patient should remain constantly *
* under the close supervision of the physician. Patients should be *
* warned to report promptly any symptoms suggesting toxicity. *
* *
************************************************************************
Penicillamine is a chelating agent used in the treatment of Wilson's disease. It is also used to reduce cystine excretion in cystinuria and to treat patients with severe, active rheumatoid arthritis unresponsive to conventional therapy (see INDICATIONS). It is 3-mercapto-D-valine. It is a white or practically white, crystalline powder, freely soluble in water, slightly soluble in alcohol, and insoluble in ether, acetone, benzene, and carbon tetrachloride. Although its configuration is D, it is levorotatory as usually measured (TABLE 1):
TABLE 1 - Penicillamine, Description
25o
(a) = -62.5o _ 2o (c = 1, 1N NaOH), calculated on a dried basis.
D
The empirical formula is C5H11NO2S, giving it a molecular weight of 149.21.
It reacts readily with formaldehyde or acetone to form a thiazolidine-carboxylic acid.
Capsules Cuprimine (Penicillamine) for oral administration contain either 125 mg or 250 mg of penicillamine. Each capsule contains the following inactive ingredients: D & C Yellow 10, gelatin, lactose, magnesium stearate, and titanium dioxide. The 125 mg capsule also contains iron oxide.
CLINICAL PHARMACOLOGY
Penicillamine is a chelating agent recommended for the removal of excess copper in patients with Wilson's disease. From in vitro studies which indicate that one atom of copper combines with two molecules of penicillamine, it would appear that one gram of penicillamine should be followed by the excretion of about 200 milligrams of copper; however, the actual amount excreted is about one percent of this.
Penicillamine also reduces excess cystine excretion in cystinuria. This is done, at least in part, by disulfide interchange between penicillamine and cystine, resulting in formation of penicillamine-cysteine disulfide, a substance that is much more soluble than cystine and is excreted readily.
Penicillamine interferes with the formation of cross-links between tropocollagen molecules and cleaves them when newly formed.
The mechanism of action of penicillamine in rheumatoid arthritis is unknown although it appears to suppress disease activity. Unlike cytotoxic immunosuppressants, penicillamine markedly lowers lgM rheumatoid factor but produces no significant depression in absolute levels of serum immunoglobulins. Also unlike cytotoxic immunosuppressants which act on both, penicillamine in vitro depresses T-cell activity but not B-cell activity.
In vitro, penicillamine dissociates macroglobulins (rheumatoid factor) although the relationship of the activity to its effect in rheumatoid arthritis is not known.
In rheumatoid arthritis, the onset of therapeutic response to Cuprimine may not be seen for two or three months. In those patients who respond, however, the first evidence of suppression of symptoms such as pain, tenderness, and swelling is generally apparent within three months. The optimum duration of therapy has not been determined. If remissions occur, they may last from months to years, but usually require continued treatment (see DOSAGE AND ADMINISTRATION).
In all patients receiving penicillamine, it is important that Cuprimine be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation by metal binding in the gastrointestinal tract.
Methodology for determining the bioavailability of penicillamine is not available; however, penicillamine is known to be a very soluble substance.
INDICATIONS
Cuprimine is indicated in the treatment of Wilson's disease, cystinuria, and in patients with severe, active rheumatoid arthritis who have failed to respond to an adequate trial of conventional therapy. Available evidence suggests that Cuprimine is not of value in ankylosing spondylitis.
Wilson's Disease--Wilson's disease (hepatolenticular degeneration) results from the interaction of an inherited defect and an abnormality in copper metabolism. The metabolic defect, which is the consequence of the autosomal inheritance of one abnormal gene from each parent, manifests itself in a greater positive copper balance than normal. As a result, copper is deposited in several organs and appears eventually to produce pathologic effects most prominently seen in the brain, where degeneration is widespread; in the liver, where fatty infiltration, inflammation, and hepatocellular damage progress to postnecrotic cirrhosis; in the kidney, where tubular and glomerular dysfunction results; and in the eye, where characteristic corneal copper deposits are known as Kayser-Fleischer rings.
Two types of patients require treatment for Wilson's disease: (1) the symptomatic, and (2) the asymptomatic in whom it can be assumed the disease will develop in the future if the patient is not treated.
Diagnosis, suspected on the basis of family or individual history, physical examination, or a low serum concentration of ceruloplasmin*, is confirmed by the demonstration of Kayser-Fleischer rings or, particularly in the asymptomatic patient, by the quantitative demonstration in a liver biopsy specimen of a concentration of copper in excess of 250 mcg/g dry weight.
Treatment has two objectives:
(1) to minimize dietary intake and absorption of copper.
(2) to promote excretion of copper deposited in tissues.
The first objective is attained by a daily diet that contains no more than one or two milligrams of copper. Such a diet should exclude, most importantly, chocolate, nuts, shellfish, mushrooms, liver, molasses, broccoli, and cereals enriched with copper, and be composed to as great an extent as possible of foods with a low copper content. Distilled or demineralized water should be used if the patient's drinking water contains more than 0.1 mg of copper per liter.
For the second objective, a copper chelating agent is used.
In symptomatic patients this treatment usually produces marked neurologic improvement, fading of Kayser-Fleischer rings, and gradual amelioration of hepatic dysfunction and psychic disturbances.
Clinical experience to date suggests that life is prolonged with the above regimen.
Noticeable improvement may not occur for one to three months. Occasionally, neurologic symptoms become worse during initiation of therapy with Cuprimine. Despite this, the drug should not be discontinued permanently, although temporary interruption may result in clinical improvement of the neurological symptoms but it carries an increased risk of developing a sensitivity reaction upon resumption of therapy (see WARNINGS).
Treatment of asymptomatic patients has been carried out for over ten years. Symptoms and signs of the disease appear to be prevented indefinitely if daily treatment with Cuprimine can be continued.
Cystinuria -- Cystinuria is characterized by excessive urinary excretion of the dibasic amino acids, arginine, lysine, ornithine, and cystine, and the mixed disulfide of cysteine and homocysteine. The metabolic defect that leads to cystinuria is inherited as an autosomal, recessive trait. Metabolism of the affected amino acids is influenced by at least two abnormal factors: (1) defective gastrointestinal absorption and (2) renal tubular dysfunction.
Arginine, lysine, ornithine, and cysteine are soluble substances, readily excreted. There is no apparent pathology connected with their excretion in excessive quantities.
Cystine, however, is so slightly soluble at the usual range of urinary pH that it is not excreted readily, and so crystallizes and forms stones in the urinary tract. Stone formation is the only known pathology in cystinuria.
Normal daily output of cystine is 40 to 80 mg. In cystinuria, output is greatly increased and may exceed 1 g/day. At 500 to 600 mg/day, stone formation is almost certain. When it is more than 300 mg/day, treatment is indicated.
Conventional treatment is directed at keeping urinary cystine diluted enough to prevent stone formation, keeping the urine alkaline enough to dissolve as much cystine as possible, and minimizing cystine production by a diet low in methionine (the major dietary precursor of cystine). Patients must drink enough fluid to keep urine specific gravity below 1.010, take enough alkali to keep urinary pH at 7.5 to 8, and maintain a diet low in methionine. This diet is not recommended in growing children and probably is contraindicated in pregnancy because of its low protein content (see PRECAUTIONS).
When these measures are inadequate to control recurrent stone formation, Cuprimine may be used as additional therapy. When patients refuse to adhere to conventional treatment, Cuprimine may be a useful substitute. It is capable of keeping cystine excretion to near normal values, thereby hindering stone formation and the serious consequences of pyelonephritis and impaired renal function that develop in some patients.
Bartter and colleagues depict the process by which penicillamine interacts with cystine to form penicillamine-cysteine mixed disulfide as (TABLE 2):
TABLE 2, Penicillamine, Indications and Uasge
CSSC + PS' ----------------« CS' + CSSP
¼---------------
PSSP + CS' ---------------« PS' + CSSP
¼---------------
CSSC + PSSP ---------------« 2 CSSP
¼---------------
CSSC = Cystine
CS' = deprotonated cysteine
PSSP = penicillamine
PS' = deprotonated penicillamine sulfhydryl
CSSP = penicillamine-cysteine mixed disulfide
In this process, it is assumed that the deprotonated form of penicillamine, PS', is the active factor in bringing about the disulfide interchange.
Rheumatoid Arthritis -- Because Cuprimine can cause severe adverse reactions, its use in rheumatoid arthritis should be restricted to patients who have severe, active disease and who have failed to respond to an adequate trial of conventional therapy. Even then, benefit-to-risk ratio should be carefully considered. Other measures, such as rest, physiotherapy, salicylates, and corticosteroids should be used, when indicated, in conjunction with Cuprimine (see PRECAUTIONS).
*For quantitative test for serum ceruloplasmin see: Morell, A. G.; Windsor, J.; Sternlieb, I.; Scheinberg, I. H.: Measurement of the concentration of ceruloplasmin in serum by determination of its oxidase activity, in "Laboratory Diagnosis of Liver Disease", F. W. Sunderman;F. W. Sunderman, Jr. (eds.), St. Louis, Warren H. Green, Inc., 1968, pp. 193-195.
CONTRAINDICATION
Except for the treatment of Wilson's disease or certain cases of cystinuria, use of penicillamine during pregnancy is contraindicated (see WARNINGS).
Although breast milk studies have not been reported in animals or humans, mothers on therapy with penicillamine should not nurse their infants.
Patients with a history of penicillamine-related aplastic anemia or agranulocytosis should not be restarted on penicillamine (see WARNINGS and ADVERSE REACTIONS).
Because of its potential for causing renal damage, penicillamine should not be administered to rheumatoid arthritis patients with a history or other evidence of renal insufficiency.
WARNING
The use of penicillamine has been associated with fatalities due to certain diseases such as aplastic anemia, agranulocytosis, thrombocytopenia, Good-pasture's syndrome, and myasthenia gravis.
Because of the potential for serious hematological and renal adverse reactions to occur at any time, routine urinalysis, white and differential blood cell count, hemoglobin determination, and direct platelet count must be done every two weeks for at least the first six months of penicillamine therapy and monthly thereafter. Patients should be instructed to report promptly the development of signs and symptoms of granulocytopenia and/or thrombocytopenia such as fever, sore throat, chills, bruising or bleeding. The above laboratory studies should then be promptly repeated.
Leukopenia and thrombocytopenia have been reported to occur in up to five percent of patients during penicillamine therapy. Leukopenia is of the granulocytic series and may or may not be associated with an increase in eosinophils. A confirmed reduction in, WBC below 3500/mm3 mandates discontinuance of penicillamine therapy. Thrombocytopenia may be on an idiosyncratic basis, with decreased or absent megakaryocytes in the marrow, when it is part of an aplastic anemia. In other cases the thrombocytopenia is presumably on an immune basis since the number of megakaryocytes in the marrow has been reported to be normal or sometimes increased. The development of a platelet count below 100,000/mm3, even in the absence of clinical bleeding, requires at least temporary cessation of penicillamine therapy. A progressive fall in either platelet count or WBC in three successive determinations, even though values are still within the normal range, likewise requires at least temporary cessation.
Proteinuria and/or hematuria may develop during therapy and may be warning signs of membranous glomerulopathy which can progress to a nephrotic syndrome. Close observation of these patients is essential. In some patients the proteinuria disappears with continued therapy; in others, penicillamine must be discontinued. When a patient develops proteinuria or hematuria the physician must ascertain whether it is a sign of drug-induced glomerulopathy or is unrelated to penicillamine.
Rheumatoid arthritis patients who develop moderate degrees of proteinuria may be continued cautiously on penicillamine therapy, provided that quantitative 24-hour urinary protein determinations are obtained at intervals of one to two weeks. Penicillamine dosage should not be increased under these circumstances. Proteinuria which exceeds 1 g/24 hours, or proteinuria which progressively increasing, requires either discontinuance of the drug or a reduction in the dosage. In some patients, proteinuria has been reported to clear following reduction in dosage.
In rheumatoid arthritis patients penicillamine should be discontinued if unexplained gross hematuria or persistent microscopic hematuria develops.
In patients with Wilson's disease or cystinuria the risks of continued penicillamine therapy in patients manifesting potentially serious urinary abnormalities must be weighed against the expected therapeutic benefits.
When penicillamine is used in cystinuria, an annual x-ray for renal stones is advised. Cystine stones form rapidly, sometimes in six months.
Up to one year or more may be required for any urinary abnormalities to disappear after penicillamine has been discontinued.
Because of rare reports of intrahepatic cholestasis and toxic hepatitis, liver function tests are recommended every six months for the duration of therapy.
Goodpasture's syndrome has occurred rarely. The development of abnormal urinary findings associated with hemoptysis and pulmonary infiltrates on x-ray requires immediate cessation of penicillamine.
Obliterative bronchiolitis has been reported rarely. The patient should be cautioned to report immediately pulmonary symptoms such as exertion, dyspnea, unexplained cough or wheezing. Pulmonary function studies should be considered at that time.
Myasthenic syndrome sometimes progressing to myasthenia gravis has been reported. Ptosis and diplopia, with weakness of the extraocular muscles are often early signs of myasthenia. In the majority of cases, symptoms of myasthenia have receded after withdrawal of penicillamine.
Most of the various forms of pemphigus have occurred during treatment with penicillamine. Pemphigus vulgaris and pemphigus foliaceus are reported most frequently, usually as a late complication of therapy. The seborrhea-like characteristics of pemphigus foliaceus may obscure an early diagnosis. When pemphigus is suspected, Cuprimine should be discontinued. Treatment has consisted of high doses of corticosteroids alone or, in some cases, concomitantly with an immunosuppressant. Treatment may be required for only a few weeks or months, but may need to be continued for more than a year.
Once instituted for Wilson's disease or cystinuria, treatment with penicillamine should, as a rule, be continued on a daily basis. Interruptions for even a few days have been followed by sensitivity reactions after reinstitution of therapy.
Use in Pregnancy -- Penicillamine has been shown to be teratogenic in rats when given in doses 6 times higher than the highest dose recommended for human use. Skeletal defects, cleft palates and fetal toxicity (resorptions) has been reported.
There are no controlled studies on the use of penicillamine in pregnant women. Although normal outcomes have been reported, characteristic congenital cutis laxa and associated birth defects have been reported in infants born of mothers who received therapy with penicillamine during pregnancy. Penicillamine should be used in women of childbearing potential only when the expected benefits outweigh the possible hazards. Women on therapy with penicillamine who are of childbearing potential should be apprised of this risk advised to report promptly any missed menstrual periods or other indications of possible pregnancy, and followed closely for early recognition of pregnancy.
Wilson's Disease -- Reported experience* shows that continued treatment with penicillamine throughout pregnancy protects the mother against relapse of the Wilson's disease, and that discontinuation of penicillamine has deleterious effects on the mother.
If penicillamine is administered during pregnancy to patients with Wilson disease, it is recommended that the daily dosage be limited to 1 g. If cesarean section is planned, the daily dosage should be limited to 250 mg during the last six weeks of pregnancy and postoperatively until wound healing is complete.
Cystinuria -- If possible, penicillamine should not be given during pregnancy to women with cystinuria (see CONTRAINDICATIONS). There are reports of women with cystinuria on therapy with penicillamine who gave birth to infants with generalized connective tissue defects who died following abdominal surgery. If stones continue to form in these patients, the benefits of therapy to the mother must be evaluated against the risk to the fetus.
Rheumatoid Arthritis -- Penicillamine should not be administered to rheumatoid arthritis patients who are pregnant (see CONTRAINDICATIONS) and should be discontinued promptly in patients in whom pregnancy is suspected or diagnosed.
There is a report that a woman with rheumatoid arthritis treated with less than one gram a day of penicillamine during pregnancy gave birth (cesarean delivery) to an infant with growth retardation, flattened face with broad nasal bridge, low set ears, short neck with loose skin folds, and unusually lax body skin.
PRECAUTIONS
Some patients may experience drug fever, a marked febrile response tp penicillamine, usually in the second to third week following initiation of therapy. Drug fever may sometimes be accompanied by a macular cutaneous eruption.
In the case of drug fever in patients with Wilson's disease or cystinuria penicillamine should be temporarily discontinued until the reaction subsides. Then penicillamine should be reinstituted with a small dose that is gradually increased until the desired dosage is attained. Systemic steroid therapy may be necessary, and is usually helpful, in such patients in whom toxic reactions develop a second or third time.
In the case of drug fever in rheumatoid arthritis patients, because other treatments are available, penicillamine should be discontinued and another therapeutic alternative tried since experience indicates that the febrile reaction will recur in a very high percentage of patients upon readministration of penicillamine.
The skin and mucous membranes should be observed for allergic reactions. Early and late rashes have occurred. Early rash occurs during the first few months of treatment and is more common. It is usually a generalized pruritic, erythematous, maculopapular or morbilliform rash and resembles the allergic rash seen with other drugs. Early rash usually disappears within days after stopping penicillamine and seldom recurs when the drug is restarted at a lower dosage. Pruritus and early rash may often be controlled by the concomitant administration of antihistamines. Less commonly, a late rash may be seen, usually after six months or more of treatment, and requires discontinuation of penicillamine. It is usually on the trunk, is accompanied by intense pruritus, and is usually unresponsive to topical corticosteroid therapy. Late rash may take weeks to disappear after penicillamine is stopped and usually recurs if the drug is restarted.
The appearance of a drug eruption accompanied by fever, arthralgia, lymphadenopathy or other allergic manifestations usually requires discontinuation of penicillamine.
Certain patients will develop a positive antinuclear antibody (ANA) test and some of these may show a lupus erythematosus-like syndrome similar to drug-induced lupus associated with other drugs. The lupus erythematosus-like syndrome is not associated with hypocomplementemia and may be present without nephropathy. The development of a positive ANA test does not mandate discontinuance of the drug; however, the physician should be alerted to the possibility that a lupus erythematosus-like syndrome may develop in the future.
Some patients may develop oral ulcerations which in some cases have the appearance of aphthous stomatitis. The stomatitis usually recurs on rechallenge but often clears on a lower dosage. Although rare, cheilosis, glossitis and gingivostomatitis have also been reported. These oral lesions are frequently dose-related and may preclude further increase in penicillamine dosage or require discontinuation of the drug.
Hypogeusia (a blunting or diminution in taste perception) has occurred in some patients. This may last two to three months or more and may develop into a total loss of taste; however, it is usually self-limited despite continued penicillamine treatment. Such taste impairment is rare in patients with Wilson's disease.
Penicillamine should not be used in patients who are receiving concurrently gold therapy, antimalarial or cytotoxic drugs, oxyphenbutazone or phenylbutazone because these drugs are also associated with similar serious hematologic and renal adverse reactions. Patients who have had gold salt therapy discontinued due to a major toxic reaction may be at greater risk of serious adverse reactions with penicillamine but not necessarily of the same type.
Patients who are allergic to penicillin may theoretically have cross-sensitivity to penicillamine. The possibility of reactions from contamination of penicillamine by trace amounts of penicillin has been eliminated now that penicillamine is being produced synthetically rather than as a degradation product of penicillin.
Because of their dietary restrictions, patients with Wilson's disease and cystinuria should be given 25 mg/day of pyridoxine during therapy, since penicillamine increases the requirement for this vitamin. Patients also may receive benefit from a multivitamin preparation, although there is no evidence that deficiency of any vitamin other than pyridoxine is associated with penicillamine. In Wilson's disease, multivitamin preparations must be copper-free.
Rheumatoid arthritis patients whose nutrition is impaired should also be given a daily supplement of pyridoxine. Mineral supplements should not be given, since they may block the response to penicillamine.
Iron deficiency may develop, especially in children and in menstruating women. In Wilson's disease, this may be a result of adding the effects of the low copper diet, which is probably also low in iron, and the penicillamine to the effects of blood loss or growth. In cystinuria, a low methionine diet may contribute to iron deficiency, since it is necessarily low in protein. If necessary, iron may be given in short courses, but a period of two hours should elapse between administration of penicillamine and iron, since orally administered iron has been shown to reduce the effects of penicillamine.
Penicillamine causes an increase in the amount of soluble collagen. In the rat this results in inhibition of normal healing and also a decrease in tensile strength of intact skin. In man this may be the cause of increased skin friability at sites especially subject to pressure or trauma, such as shoulders, elbows, knees, toes, and buttocks. Extravasations of blood may occur and may appear as pupuric areas, with external bleeding if the skin is broken, or as vesicles containing dark blood. Neither type is progressive. There is no apparent association with bleeding elsewhere in the body and no associated coagulation defect has been found. Therapy with penicillamine may be continued in the presence of these lesions. They may not recur if dosage is reduced. Other reported effects probably due to the action of penicillamine on collagen are excessive wrinkling of the skin and development of small, white papules at venipuncture and surgical sites.
The effects of penicillamine on collagen and elastin make it advisable to consider a reduction in dosage to 250 mg/day, when surgery is contemplated. Reinstitution of full therapy should be delayed until wound healing is complete.
Carcinogenesis -- Long-term animal carcinogenicity studies have not been done with penicillamine. There is a report that five of ten autoimmune disease-prone NZB hybrid mice developed lymphocytic leukemia after 6 months' intraperitoneal treatment with a dose of 400 mg/kg penicillamine 5 days per week.
Nursing Mothers -- See CONTRAINDICATIONS.
Usage in Children -- The efficacy of Cuprimine in juvenile rheumatoid arthritis has not been established.
*Scheinberg, I.H.; Sternlieb, I.: N. Engl. J. Med. 293: 1300-1302, Dec. 18, 1975
PENICILLAMINE
ADVERSE REACTIONS
Penicillamine is a drug with a high incidence of untoward reactions, some of which are potentially fatal. Therefore, it is mandatory that patients receiving penicillamine therapy remain under close medical supervision throughout the period of drug administration (see WARNINGS and PRECAUTIONS).
Reported incidences (%) for the most commonly occurring adverse reactions in rheumatoid arthritis patients are noted, based on 17 representative clinical trials reported in the literature (1270 patients).
Allergic -- Generalized pruritus, early and late rashes (5%), pemphigus (see WARNINGS), and drug eruptions which may be accompanied by fever, arthralgia, or lymphadenopathy have occurred (see WARNINGS and PRECAUTIONS). Some patients may show a lupus erythematosus-like syndrome similar to drug-induced lupus produced by other pharmacological agents (see PRECAUTIONS).
Urticaria and exfoliative dermatitis have occurred.
Thyroiditis has been reported; hypoglycemia in association with anti-insulin antibodies has been reported. These reactions are extremely rare.
Some patients may develop a migratory polyarthralgia, often with objective synovitis (see DOSAGE AND ADMINISTRATION).
Gastrointestinal -- Anorexia, epigastric pain, nausea, vomiting, or occasional diarrhea may occur (17%).
Isolated cases of reactivated peptic ulcer have occurred, as have hepatic dysfunction and pancreatitis. Intrahepatic cholestasis and toxic hepatitis have been reported rarely. There have been a few reports of increased serum alkaline phosphatase, lactic dehydrogenase, and positive cephalin flocculation and thymol turbidity tests.
Some patients may report a blunting, diminution, or total loss of taste perception (12%); or may develop oral ulcerations. Although rare, cheilosis, glossitis, and gingivostomatitis have been reported (see PRECAUTIONS).
Gastrointestinal side effects are usually reversible following cessation of therapy.
Hematological -- Penicillamine can cause bone marrow depression (see WARNINGS). Leukopenia (2%) and thrombocytopenia (4%) have occurred. Fatalities have been reported as a result of thrombocytopenia, agranulocytosis, aplastic anemia, and sideroblastic anemia.
Thrombotic thrombocytopenic purpura, hemolytic anemia, red cell aplasia, monocytosis, leukocytosis, eosinophilia, and thrombocytosis have also been reported.
Renal -- Patients on penicillamine therapy may develop proteinuria (6%) and/or hematuria which, in some, may progress to the development of the nephrotic syndrome as a result of an immune complex membranous glomerulopathy (see WARNINGS).
Central Nervous System -- Tinnitus, optic neuritis and peripheral sensory and motor neuropathies (including polyradiculoneuropathy, i.e., Guillain-Barreé syndrome) have been reported. Muscular weakness may or may not occur with the peripheral neuropathies. Visual and psychic disturbances have been reported.
Neuromuscular -- Myasthenia gravis (see WARNINGS).
Other -- Adverse reactions that have been reported rarely include thrombophlebitis; hyperpyrexia (see PRECAUTIONS); falling hair or alopecia; lichen planus; polymyositis; dermatomyositis; mammary hyperplasia; elastosis perforans serpiginosa; toxic epidermal necrolysis; anetoderma (cutaneous macular atrophy); and Goodpasture's syndrome, a severe and ultimately fatal glomerulonephritis associated with intra-alveolar hemorrhage (see WARNINGS). Fatal renal vasculitis has also been reported. Allergic alveolitis, obliterative bronchiolitis, interstitial pneumonitis and pulmonary fibrosis have been reported in patients with severe rheumatoid arthritis, some of whom were receiving penicillamine. Bronchial asthma also has been reported.
Increased skin friability, excessive wrinkling of skin, and development of small white papules at venipuncture and surgical sites have been reported (see PRECAUTIONS).
The chelating action of the drug may cause increased excretion of other heavy metals such as zinc, mercury and lead.
There have been reports associating penicillamine with leukemia. However, circumstances involved in these reports are such that a cause and effect relationship to the drug has not been established.
DOSAGE AND ADMINISTRATION
In all patients receiving penicillamine, it is important that Cuprimine be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food, or milk. Because penicillamine increases the requirement for pyridoxine, patients may require a daily supplement of pyridoxine (see PRECAUTIONS).
Wilson's Disease -- Optimal dosage can be determined by measurement of urinary copper excretion and the determination of free copper in the serum. The urine must be collected in copper-free glassware, and should be quantitatively analyzed for copper before and soon after initiation of therapy with Cuprimine.
Determination of 24-hour urinary copper excretion is of greatest value in the first week of therapy with penicillamine. In the absence of any drug reaction, a dose between 0.75 and 1.5 g that results in an initial 24-hour cupriuresis of over 2 mg should be continued for about three months, by which time the most reliable method of monitoring maintenance treatment is the determination of free copper in the serum. This equals the difference between quantitatively determined total copper and ceruloplasmin-copper. Adequately treated patients will usually have less than 10 mcg free copper/dl of serum. It is seldom necessary to exceed a dosage of 2 g/day. If the patient is intolerant to therapy with Cuprimine, alternative treatment is trientine hydrochloride.
In patients who cannot tolerate as much as 1 g/day initially, initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions.
Cystinuria -- It is recommended that Cuprimine be used along with conventional therapy. By reducing urinary cystine, it decreases crystalluria and stone formation. In some instances, it has been reported to decrease the size of, and even to dissolve, stones already formed.
The usual dosage of Cuprimine in the treatment of cystinuria is 2 g/day for adults, with a range of 1 to 4 g/day. For children, dosage can be based on 30 mg/kg/day. The total daily amount should be divided into four doses. If four equal doses are not feasible, give the larger portion at bedtime. If adverse reactions necessitate a reduction in dosage, it is important to retain the bedtime dose.
Initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions.
In addition to taking Cuprimine, patients should drink copiously. It is especially important to drink about a pint of fluid at bedtime and another pint once during the night when urine is more concentrated and more acid than during the day. The greater the fluid intake, the lower the required dosage of Cuprimine.
Dosage must be individualized to an amount that limits cystine excretion to 100-200 mg/day in those with no history of stones, and below 100 mg/day in those who have had stone formation and/or pain. Thus, in determining dosage, the inherent tubular defect, the patient's size, age, and rate of growth, and his diet and water intake all must be taken into consideration.
The standard nitroprusside cyanide test has been reported useful as a qualitative measure of the effective dose*: Add 2 ml of freshly prepared 5 percent sodium cyanide to 5 ml of a 24-hour aliquot of protein-free urine and let stand ten minutes. Add 5 drops of freshly prepared 5 percent sodium nitroprusside and mix. Cystine will turn the mixture magenta. If the result is negative, it can be assumed that cystine excretion is less than 100 mg/g creatinine.
Although penicillamine is rarely excreted unchanged, it also will turn the mixture magenta. If there is any question as to which substance is causing the reaction, a ferric chloride test can be done to eliminate doubt: Add 3 percent ferric chloride dropwise to the urine. Penicillamine will turn the urine an immediate and quickly fading blue. Cystine will not produce any change in appearance.
Rheumatoid Arthritis -- The principal rule of treatment with Cuprimine in rheumatoid arthritis is patience. The onset of therapeutic response is typically delayed. Two or three months may be required before the first evidence of a clinical response is noted (see CLINICAL PHARMACOLOGY).
When treatment with Cuprimine has been interrupted because of adverse reactions or other reasons, the drug should be reintroduced cautiously by starting with a lower dosage and increasing slowly.
Initial Therapy -- The currently recommended dosage regimen in rheumatoid arthritis begins with a single daily dose of 125 mg or 250 mg which is thereafter increased at one to three month intervals, by 125 mg or 250 mg/day, as patient response and tolerance indicate. If a satisfactory remission of symptoms is achieved, the dose associated with the remission should be continued (see Maintenance Therapy). If there is no improvement and there are no signs of potentially serious toxicity after two to three months of treatment with doses of 500-750 mg/day, increases of 250 mg/day at two to three month intervals may be continued until a satisfactory remission occurs (see Maintenance Therapy) or signs of toxicity develop (see WARNINGS and PRECAUTIONS). If there is no discernible improvement after three to four months of treatment with 1000 to 1500 mg of penicillamine/day, it may be assumed the patient will not respond and Cuprimine should be discontinued.
Maintenance Therapy -- The maintenance dosage of Cuprimine must be individualized, and may require adjustment during the course of treatment. Many patients respond satisfactorily to a dosage within the 500-750 mg/day range. Some need less.
Changes in maintenance dosage levels may not be reflected clinically or in the erythrocyte sedimentation rate for two to three months after each dosage adjustment.
Some patients will subsequently require an increase in the maintenance dosage to achieve maximal disease suppression. In those patients who do respond, but who evidence incomplete suppression of their disease after the first six to nine months of treatment, the daily dosage of Cuprimine may be increased by 125 mg or 250 mg/day at three-month intervals. It is unusual in current practice to employ a dosage in excess of 1 g/day, but up to 1.5 g/day has sometimes been required.
Management of Exacerbations -- During the course of treatment some patients may experience an exacerbation of disease activity following an initial good response. These may be self-limited and can subside within twelve weeks. They are usually controlled by the addition of non-steroidal anti-inflammatory drugs, and only if the patient has demonstrated a true "escape" phenomenon (as evidenced by failure of the flare to subside within this time period) should an increase in the maintenance dose ordinarily be considered.
In the rheumatoid patient, migratory polyarthralgia due to penicillamine is extremely difficult to differentiate from an exacerbation of the rheumatoid arthritis. Discontinuance or a substantial reduction in dosage of Cuprimine for up to several weeks will usually determine which of these processes is responsible for the arthralgia.
Duration of Therapy -- The optimum duration of therapy with Cuprimine in rheumatoid arthritis has not been determined. If the patient has been in remission for six months or more, a gradual, stepwise dosage reduction in decrements of 125 mg or 250 mg/day at approximately three month intervals may be attempted.
Concomitant Drug Therapy -- Cuprimine should not be used in patients who are receiving gold therapy, antimalarial or cytotoxic drugs, oxyphenbutazone, or phenylbutazone (see PRECAUTIONS). Other measures, such as salicylates, other non-steroidal anti-inflammatory drugs, or systemic corticosteroids, may be continued when penicillamine is initiated. After improvement commences, analgesic and anti-inflammatory drugs may be slowly discontinued as symptoms permit. Steroid withdrawal must be done gradually, and many months of treatment with Cuprimine may be required before steroids can be completely eliminated.
Dosage Frequency -- Based on clinical experience dosages up to 500 mg/day can be given as a single daily dose. Dosages in excess of 500 mg/day should be administered in divided doses.
Storage
Keep container tightly closed.
* Lotz, M.; Potts, J. T. and Bartter, F. C.: Brit. Med. J. 2: 521, Aug. 28, 1965 (in Medical Memoranda).
(Merck, 3/89,741238)
(89/03)
PENICILLAMINE
HOW SUPPLIED - EQUIVALENTS NOT AVAILABLE:
Capsule, Gelatin - Oral - 125 mg
100's $64.88 CUPRIMINE, Merck 00006-0672-68*
Capsule, Gelatin - Oral - 250 mg
100's $92.63 CUPRIMINE, Merck 00006-0602-68*
Tablet, Coated - Oral - 250 mg
100's $148.24 DEPEN TITRATABLE, Carter-Wallace 00037-4401-01*
======================================================================
======================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.0024
Tlf: 0414-2976087 - 04127766810















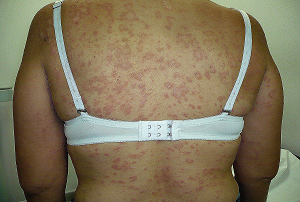



.png)