ACICLOVIR, VALACICLOVIR Y EMBARAZO
Para la década de los años 80, fue desarrollada la molécula ACICLOVIR por el laboratorio Burroughs Wellcome & Company (hoy GlaxoSmithKline), primeramente en ungüento para el tratamiento del Herpes Simple tipos I y II, y posteriormente fue aprobado por la FDA, en tabletas de 200 mgrs, el nombre comercial ORIGINAL fue ZOVIRAX.
El ACICLOVIR tiene una biodisponibilidad muy baja, 10 al 30% y tiempo de vida corto, 3 A 5 HORAS, por lo cual hay que administrarlo entre 3 y 5 veces al día, por ello luego salieron al mercado presentaciones de 400 y 800 Mgrs.
Posteriormente el mismo laboratorio en la década de los 90, patenta el VALACICLOVIR, el cual es una PRO DROGA, es decir que al administrarlo se convierte en ACICLOVIR, pero su biodiponibilidad es mayor, (55%), lo que permite administrarlo en mayor lapso de tiempo, es decir cada 12 horas.
La presentación en tabletas es de 500 Mgrs, y originalmente se le conoce con el nombre de VALTREX, no hay presentación en ungüento, a diferencia de su predecesor el ACICLOVIR.
USOS:
Ambos MEDICAMENTOS, son utilizados hoy día en:
- HERPES SIMPLE I y II.
- VARICELA (Virus de la Varicela- Zoster)
- HERPES ZÓSTER
- QUERATITIS HERPÉTICA.
- HERPES en pacientes INMUNODEPRIMIDOS.
- PITIRIASIS ROSADA (Herpes 6 y 7).
- CITOMEGALOVIRUS (CMV).
- VIRUS DE EPSTEIN BARR (EBV).
- HERPES SIMPLE, ZOSTER, CITOMEGALOVIRUS, y VARICELA en el EMBARAZO.
Lo mas importante de esta revisión, es que estas MOLÉCULAS, que tienen mas de 30 años en el mercado, hoy dia 2025, siguen utilizándose en estas patologías, Y SOBRE TODO son SEGURAS en el embarazo, no teniendo efectos secundarios, tanto para la madre como para el feto.
Múltiples estudios han demostrado seguridad del VALACICLOVIR en el primer trimestre del embarazo, y tampoco se han reportado MALFORMACIONES CONGÉNITAS.
Saludos,,,
Dr. José Lapenta.
ENGLISH
In the 1980s, the ACICLOVIR molecule was developed by Burroughs Wellcome & Company (now GlaxoSmithKline), initially as an ointment for the treatment of Herpes Simplex types I and II. It was later approved by the FDA in 200 mg tablets. The original brand name was ZOVIRAX.
ACICLOVIR has a very low bioavailability (10% to 30%) and a short half-life (3 to 5 hours). Therefore, it must be administered 3 to 5 times a day. For this reason, 400 and 800 mg formulations later came onto the market.
Later, in the 1990s, the same laboratory patented VALACICLOVIR, which is a prodrug, meaning that when administered, it converts to acyclovir, but its bioavailability is greater (55%), allowing it to be administered over a longer period of time, i.e., every 12 hours.
The tablet form is 500 mg and was originally known as VALTREX; it does not come in an ointment form, unlike its predecessor, acyclovir.
USES:
Both medications are used today for:
- HERPES SIMPLEX TYPE I and II.
- CHICKENPOX (Varicella-Zoster Virus).
- HERPES ZOSTER (Shingles).
- HERPETIC KERATITIS.
- HERPES IN IMMUNOSUPPRESSED PATIENTS.
- PITYRIASIS ROSEA (Herpes Types 6 and 7).
- CYTOMEGALOVIRUS (CMV).
- EPSTEIN-BARR VIRUS (EBV).
- HERPES SIMPLEX, ZOSTER, CITOMEGALOVIRUS, AND CHICKENPOX IN PREGNANCY.
The most important aspect of this review is that these molecules, which have been on the market for more than 30 years, continue to be used for these diseases today (in 2025), and above all, they are SAFE during pregnancy, with no side effects for either the mother or the fetus.
Multiple studies have demonstrated the safety of VALACICLOVIR in the first trimester of pregnancy, and no birth defects have been reported.
Greetings...
Dr. José Lapenta R.
EDITORIAL ESPANOL:
====================
Hola amigos de la red, DERMAGIC de nuevo con ustedes. El tema, el ACICLOVIR, y EMBARAZO.
Esta popular medicina con unos cuantos años en el mercado se le ha encontrado efectividad contra los virus del Herpes Simple, Varicela Zoster,(VZB), Epstein Barr (EBV), Citomegalovirus (CMV) y Herpes Virus 6 (HHV-6).
Todavía en nuestros días resulta de gran utilidad contra algunas de estas enfermedades virales sobre todo en el embarazo donde ha mostrado ser bastante segura para la madre y feto y con una buena efectividad en casos de Herpes Simple, Zoster y Varicela.
De modo que aun con la aparición de nuevas drogas contra estos virus como el: Famciclovir, Valaciclovir Ganciclovir y Cidofovir, el ACICLOVIR ocupa todavía un lugar importante como arma terapéutica contra estos agentes virales.
Algunos Dermatólogos también han utilizado el ACICLOVIR, en la Pitiriasis Rosada de Gibert pensando en una posible causa viral (herpes virus 6, 7), y también en la Pitiriasis Liquenoide Varioliforme Aguda (Enfermedad de Mucha-Habermann), cuando hay infecciones por herpes virus asociados.
Saludos,,,
Dr. José Lapenta R.,,,
EDITORIAL ENGLISH:
===================
Hello friends of the net, DERMAGIC again with you. The topic, the ACICLOVIR, and PREGNANCY.
This popular medicine with some years in the market has been found effectiveness against the Herpes Simplex Viruses, Varicella-Zoster Virus,(VZB), Epstein Barr Virus (EBV), Cytomegalovirus (CMV) and Herpes Virus 6 (HHV-6).
Still in our days it is mainly of great utility against some of these viral illnesses in the pregnancy where it has shown to be quite safe for the mother and fetus and with a good effectiveness in cases of Herpes Simplex, Zoster and Varicella.
So that even with the appearance of new drugs against these viruses like the one: Famciclovir, Valaciclovir Ganciclovir and Cidofovir, the ACICLOVIR still occupies an important place as weapon therapy against these viral agents.
Some Dermatologist the ACICLOVIR has also used, in the Pityriasis Rosea of Gibert thinking of a possible viral cause (herpes virus 6, 7), and others like the Pityriasis Lichenoid et Varioliformis Acuta. (Mucha Habermann disease), when there are associated herpes virus.
Greetings,,,
Dr. José Lapenta R.
================================================================
REFERENCIAS BIBLIOGRÁFICAS / BIBLIOGRAPHICAL REFERENCES
================================================================
C.-Herpes Zoster and Postherpetic Neuralgia: Prevention and Management.
D- Herpes Zoster: A Brief Definitive Review.
E.-Advances and Perspectives in the Management of Varicella-Zoster Virus.
=================================================================
1.)Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy.
2.) Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy.
3.) Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy.
4.) Uses and safety of acyclovir in pregnancy.
5.) Pharmacokinetics of acyclovir in the term human pregnancy and neonate.
6.) Systemic acyclovir in pregnancy: a case report.
7.) Treatment of disseminated herpes simplex virus in pregnancy with parenteral acyclovir. A case report.
8.) [Recurrent cutaneous herpes in the newborn and acyclovir].
9.) Oral acyclovir and recurrent genital herpes during late pregnancy.
10.) Acyclovir therapy during pregnancy.
11.) Herpes simplex virus hepatitis in pregnancy. A case report.
12.) Disseminated herpes simplex infection in an immunocompromised pregnancy: treatment with intravenous acyclovir.
13.) Acyclovir for disseminated herpes simplex virus in pregnancy. A case report.
14.) Acyclovir for the prevention and treatment of varicella zoster in children, adolescents and pregnancy.
15.) Treatment with acyclovir of varicella pneumonia in pregnancy.
16.) Varicella pneumonia during pregnancy. Treatment of two cases with acyclovir.
17.) Use of acyclovir for varicella pneumonia during pregnancy.
18.) Varicella during pregnancy. Maternal and fetal effects.
19.) Perinatal outcome of pregnancies complicated with varicella
20.) Varicella and pregnancy.
21.) Intrauterine infection with varicella-zoster virus after maternal varicella.
22.) [Varicella in pregnancy after the 20th week of amenorrhea].
23.) Varicella in pregnancy.
24.) [Chickenpox and pregnancy. Perinatal aspects and prevention].
25.) Use of acyclovir for varicella pneumonia during pregnancy.
26.) Fatal disseminated herpes simplex in pregnancy with maternal and neonatal death.
27.) Connatal herpes zoster.
28.) Pregnancy outcomes following systemic prenatal acyclovir exposure--June 1, 1984-June 30, 1993.
29.) Severe pneumonia in pregnancy three months after resolution of cutaneous zoster.
30.) Disseminated herpes zoster in a pregnant woman positive for human immunodeficiency virus.
31.) Early-second-trimester use of acyclovir in treating herpes zoster in a bone marrow transplant patient. A case report.
32.) [Acyclovir and pregnancy: current aspects].
33.) [A case of delayed cerebral infarction occurring in puerperium preceded by herpes zoster ophthalmicus in late pregnancy].
34.) Congenital varicella-zoster virus infection and Barrett's esophagus.
35.) Antibodies to varicella zoster virus in the cerebrospinal fluid of neonates with seizures.
36.) ACYCLOVIR (Systemic), The product
=================================================================== ===================================================================
1.)Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy.
===================================================================
Drugs 1994 Jan;47(1):153-205
Wagstaff AJ, Faulds D, Goa KL
Adis International Limited, Auckland, New Zealand.
Aciclovir (acyclovir) is a nucleoside analogue with antiviral activity in vitro against the herpes simplex viruses (HSV), varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV) and human herpesvirus 6 (HHV-6). Topical, oral or intravenous aciclovir is well
established in the treatment of ophthalmic, mucocutaneous and other HSV infections, with intravenous aciclovir the accepted treatment of choice in herpes simplex encephalitis. The efficacy of aciclovir is increased with early (preferably during the prodromal period) initiation of treatment but, despite significant clinical benefit, viral latency is not eradicated, and pretreatment frequencies of recurrence usually continue after episodic acute treatment is completed. Intravenous administration has also shown benefit in the treatment of severe complications of HSV infection in pregnancy, and neonatal HSV infections.
Recurrence of HSV has been completely prevented or significantly reduced during suppressive therapy with oral aciclovir in immunocompetent patients. Use of oral aciclovir is effective but controversial in the treatment of otherwise healthy individuals with varicella (chickenpox), and in some countries it has been recommended for use only in cases which may be potentially severe. The development of rash and pain associated with herpes zoster (shingles) is attenuated with oral or intravenous aciclovir therapy, ocular involvement is prevented, and post-herpetic neuralgia appears to be decreased.
Similarly, in a few patients with zoster ophthalmicus, oral aciclovir has reduced the frequency and severity of long term ocular complications and post-herpetic neuralgia, and herpes zoster oticus is improved with intravenous aciclovir. Oral aciclovir has prevented recurrence of HSV genital or orofacial infections during suppressive therapy in > 70% of immunocompetent patients in most clinical trials.
Suppression of latent HSV, VZV and CMV infections has been achieved in many immunocompromised patients receiving the oral or intravenous formulations. Aciclovir also appears to offer partial protection from invasive CMV disease in CMV-seropositive bone marrow transplant recipients. The few comparative trials published have shown aciclovir to be at least as effective as other investigated antivirals in the treatment of HSV infections in immunocompetent patients, and more effective than inosine pranobex in the prophylaxis of genital herpes. Similarly, in isolated clinical trials, oral aciclovir appears as effective as topical idoxuridine and oral brivudine in some parameters in immunocompetent patients with VZV infections, and the intravenous formulation appears at least as effective as oral brivudine and intravenous vidarabine in treating these infections in immunocompromised patients.
===================================================================
2.) Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy.
===================================================================
Drugs 1989 Mar;37(3):233-309
O'Brien JJ, Campoli-Richards DM
ADIS Drug Information Services, Auckland, New Zealand.
Acyclovir (aciclovir) is a nucleoside antiviral drug with antiviral activity in vitro against members of the herpes group of DNA viruses. As an established treatment of herpes simplex infection, intravenous, oral and to a lesser extent topical formulations of acyclovir provide significant therapeutic benefit in genital herpes simplex and recurrent orofacial herpes simplex.
The effect of acyclovir therapy is maximised by early initiation of treatment, especially in non-primary infection which tends to have a less protracted course than the primary episode. Long term prophylactic oral acyclovir, in patients with frequent episodes of genital herpes simplex, totally suppresses recurrences in the majority of subjects; as with other infections responding to acyclovir, viral latency is not eradicated and pretreatment frequencies of recurrence return after discontinuation of treatment.
Caution should accompany the prophylactic use of acyclovir in the general population, due to the theoretical risk of the emergence of viral strains resistant to acyclovir and other agents whose mechanism of action is dependent on viral thymidine kinase. Intravenous acyclovir is the treatment of choice in biopsy-proven herpes simplex encephalitis in adults, and has also been successful in the treatment of disseminated herpes simplex in pregnancy and herpes neonatorium.
Intravenous and oral acyclovir protect against dissemination and progression of varicella zoster virus infection, but do not protect against post-herpetic neuralgia. In immunocompromised patients, intravenous, oral and topical acyclovir shorten the clinical course of herpes simplex infections while prophylaxis with oral or intravenous dosage forms suppresses reactivation of infection during the period of drug administration. Ophthalmic application of 3% acyclovir ointment rapidly heals herpetic dendritic corneal ulcers and superficial herpetic keratitis.
Thus, despite an inability to eradicate latent virus, acyclovir administered in therapeutic or prophylactic fashion is now the standard antiviral therapy in several manifestations of herpes simplex virus infection, and indeed represents a major advance in this regard. With the exception of varicella zoster virus infections, early optimism concerning the use of the drug in diseases due to other herpes viruses has generally not been supported in clinical investigations.
===================================================================
3.) Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy.
===================================================================
Drugs 1983 Nov;26(5):378-438
Richards DM, Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS
Acyclovir (aciclovir) is a nucleoside analogue antiviral drug related to cytarabine, idoxuridine, trifluridine and vidarabine. In common with these earlier antivirals, acyclovir is active against some members of the herpesvirus group of DNA viruses. The efficacy of topical acyclovir has been convincingly demonstrated in ocular herpetic keratitis, and in initial and primary initial genital herpes infection, but little or no clinical benefit was seen when non-primary initial genital infections were assessed separately.
Acyclovir ointment demonstrated little benefit in recurrent genital herpes but topical acyclovir cream decreased the course of the infection by 1 to 2 days. Orally and intravenously administered acyclovir were beneficial in initial genital herpes infections, and oral therapy shortened the duration of recurrent infections by 1 to 2 days but did not ameliorate pain. In non-immunocompromised patients with recurrent herpes simplex labialis, generally little clinical benefit was seen with the use of topical acyclovir ointment even when therapy was initiated during the prodromal phase, while topical acyclovir cream effected small but significant improvements in the clinical but not the symptomological course of the disease.
However, in immunocompromised patients, both intravenous and topical acyclovir shortened the clinical course of herpes simplex virus infections occurring mainly on the lips, oral mucosa and face, and prophylaxis with either oral or intravenous acyclovir suppressed the appearance of recurrent lesions from latent virus for the period of drug administration, but acyclovir did not eradicate latent herpesviruses. In non-immunocompromised patients, intravenous acyclovir was shown to decrease the acute pain of zoster, especially in the elderly, but postherpetic neuralgia was not ameliorated.
When immunocompromised patients were studied, intravenous acyclovir inhibited the progression of zoster infections and shortened the healing time and duration of viral shedding in patients with cutaneous disseminated zoster. However, acute and post-herpetic pain were not significantly affected. Well designed controlled studies are underway to establish the efficacy of acyclovir in herpes simplex encephalitis and cytomegalovirus infections in immunocompromised patients, infections due to Epstein-Barr virus, and neonatal herpesvirus infections.
Despite some aspects of the drug's use which require further clarification, acyclovir will make a major impact on the treatment of herpesviral infections. Barring unexpected findings with wider clinical use, it will become the agent of choice in several conditions.
===================================================================
4.) Uses and safety of acyclovir in pregnancy.
===================================================================
J Fam Pract 1994 Feb;38(2):186-91
Spangler JG, Kirk JK, Knudson MP
Department of Family and Community Medicine, Bowman Gray School of Medicine of Wake Forest University, Winston-Salem, NC 27157-1084.
Acyclovir, an antiviral nucleoside analogue, is a widely used agent highly specific for herpes simplex and varicella-zoster viruses. Unintended exposure to acyclovir early in pregnancy, which is not uncommon, may cause excessive maternal and physician anxiety.
This drug has not been studied prospectively in large numbers of pregnant women and lacks the Food and Drug Administration's approval for gestational use unless benefits clearly outweigh potential fetal harm. However, data published since acyclovir became available do not indicate increased adverse effects related to its use in pregnancy, especially if prescribed in selected situations, such as disseminated primary herpes simplex infections or maternal varicella pneumonia. This article reports the impact of inadvertent acyclovir exposure on a woman during the first trimester of pregnancy and reviews the literature on acyclovir's pharmacology, safety profile, and potential uses during pregnancy.
===================================================================
5.) Pharmacokinetics of acyclovir in the term human pregnancy and neonate.
===================================================================
Am J Obstet Gynecol 1991 Feb;164(2):569-76
Frenkel LM, Brown ZA, Bryson YJ, Corey L, Unadkat JD, Hensleigh PA, Arvin AM, Prober CG, Connor JD
Department of Pediatrics, University of California, Los Angele Center for Health Sciences 90024-1752.
Concern about neonatal herpes often leads to cesarean delivery of infants in women with a history of genital herpes. The antiviral drug acyclovir has been used effectively to suppress genital herpes simplex virus recurrences in nonpregnant adults. Its administration to pregnant women with recurrent genital herpes may reduce herpes simplex virus recurrences and thus may decrease the cesarean section rate among this population. To study the pharmacokinetics, safety, and patient tolerance of suppressive oral acyclovir, either 200 mg (n = 7) or 400 mg (n = 8) was administered orally every 8 hours to pregnant women with a history of recurrent herpes simplex virus, from 38 weeks' gestation until delivery.
The mean +/- SD plasma levels for the 200 and 400 mg groups, respectively, were: first dose peak, 1.7 +/- 0.6 and 2.3 +/- 1.0 mumol/L; steady-state trough, 0.7 +/- 0.3 and 0.8 +/- 0.6 mumol/L; steady-state peak, 1.9 +/- 1.0 and 3.3 +/- 1.0 mumol/L. In late gestation maternal acyclovir pharmacokinetics were similar to those of nonpregnant adults from other studies. Acyclovir was concentrated in the amniotic fluid; however, there was no accumulation in the fetus (mean maternal/infant plasma ratio at delivery was 1.3).
Acyclovir was well tolerated, and no toxicity was seen in the mothers or infants. The administration of acyclovir, 400 mg every 8 hours, appears appropriate for use in an efficacy and safety study regarding suppression of herpes simplex virus recurrences during the last weeks of pregnancy.
===================================================================
6.) Systemic acyclovir in pregnancy: a case report.
===================================================================
Obstet Gynecol 1985 Feb;65(2):284-7
Grover L, Kane J, Kravitz J, Cruz A
Disseminated herpes simplex infection in pregnancy presents serious risk to mother and fetus. Although an uncommon problem, the high maternal and fetal mortality and morbidity accompanying disseminated herpes infection warrants aggressive new treatment. Specific antiviral chemotherapy is now possible for selected cases. The present report describes the use of acyclovir during the third trimester for disseminated herpes simplex infection. The treatment protocol used and pregnancy outcome are described for this case. Acyclovir therapy and potential toxicities are described.
===================================================================
7.) Treatment of disseminated herpes simplex virus in pregnancy with parenteral acyclovir. A case report.
===================================================================
J Reprod Med 1986 Oct;31(10):1005-7
Cox SM, Phillips LE, DePaolo HD, Faro S
Disseminated herpes simplex virus infection in a pregnant woman was successfully treated with acyclovir. Similar reported cases have suggested that acyclovir may be suitable for the treatment of disseminated or severe primary herpes in pregnant women.
===================================================================
8.) [Recurrent cutaneous herpes in the newborn and acyclovir].
===================================================================
Pediatrie 1993;48(5):381-3
Haddad J, Pierrat V, Langer B, Rousseau S, Astruc D, Messer J, Lequien P
Service de neonatologie, CHU de Hautepierre, Strasbourg, France.
The authors report two cases of cutaneous recurrent herpes occurring after a neonatal herpes simplex virus type 2 (HSV2) infection and comment on the role of acute or suppressive therapy by aciclovir (ACV). The two infants were not treated by ACV after the neonatal period. None of the recurrent cutaneous herpes episodes was followed by viral widespread.
One case reported by Bergstrom et al on a relapse of HSV2 encephalitis occurring after a cutaneous herpes in a child argues for the use of ACV in recurrent herpes. However, ACV might alter host defense response to HSV2 infection in neonates and children. Thus, it seems not yet recommended to use ACV either as acute or suppressive therapy in recurrent cutaneous herpes unless a progression of the viral disease is noted.
===================================================================
9.) Oral acyclovir and recurrent genital herpes during late pregnancy.
===================================================================
Obstet Gynecol 1993 Jul;82(1):102-4
Haddad J, Langer B, Astruc D, Messer J, Lokiec F
Service de Neonatologie, Hospital University of Strasbourg, France.
OBJECTIVE: To assess plasma acyclovir levels in pregnant women given oral acyclovir during late gestation and to determine the role and effect of oral acyclovir on asymptomatic shedding of virus in cases of recurrent genital herpes.
METHODS: Five pregnant women with proven genital herpes isolate (herpes simplex virus [HSV] 2) after 37 weeks' gestation were studied. Oral acyclovir was administered every 8 hours at dosages of 300, 400, and 300 mg in two subjects, and 200 mg five times daily in the other three until delivery. Plasma acyclovir peak and trough levels were determined. Viral cultures were obtained from both the mothers and neonates at delivery.
RESULTS: There was no difference in acyclovir plasma levels among the patients. Furthermore, acyclovir levels were comparable to those of nonpregnant adults. The drug failed to suppress asymptomatic shedding of virus and transmission of HSV 2 to the neonate in one of five of the patients.
CONCLUSION: Our study suggests that asymptomatic shedding of virus is not prevented by use of oral acyclovir during late gestation in proven recurrent genital herpes even though plasma acyclovir levels were within the normal range.
===================================================================
10.) Acyclovir therapy during pregnancy.
===================================================================
Obstet Gynecol 1989 Mar;73(3 Pt 2):526-31
Brown ZA, Baker DA
Department of Obstetrics and Gynecology, University of Washington, Seattle.
Although there are as yet no established indications for acyclovir use in pregnancy, the most reasonable uses are for maternal infections such as disseminated herpes simplex, varicella pneumonia, and severe primary genital herpes.
Other potential, but more problematic, uses during pregnancy are for uncomplicated primary genital herpes infections, maternal varicella, and for prophylaxis against the recurrence of genital herpes near term. We review each of these potential uses and the pharmacokinetics of acyclovir in pregnancy while emphasizing that at the present time, safety, efficacy, and appropriate dosage of the drug have not been established for any use in pregnancy.
===================================================================
11.) Herpes simplex virus hepatitis in pregnancy. A case report.
===================================================================
J Reprod Med 1994 Jul;39(7):544-6
Johnson LG, Saldana LR
Department of Obstetrics and Gynecology, Bethesda Hospital, Cincinnati, Ohio.
Hepatitis is a rare but serious complication of a herpes simplex viral infection. Pregnancy is a risk factor and has been associated with a high maternal and fetal mortality rate. Acyclovir appears to improve the outcome.
===================================================================
12.) Disseminated herpes simplex infection in an immunocompromised pregnancy: treatment with intravenous acyclovir.
===================================================================
Am J Perinatol 1987 Oct;4(4):363-4
Chazotte C, Andersen HF, Cohen WR
Department of Obstetrics and Gynecology, Bronx Municipal Hospital Center, Albert Einstein College of Medicine, New York.
In this article, we report a case of third-trimester disseminated herpes simplex virus (HSV) infection in an immunocompromised gravida who was treated with parenteral acyclovir. Rapid resolution of lesions occurred, and the fetus was delivered at term without evident abnormalities. Of the four previous reports on this therapy, there has been one maternal death and survival of all neonates. Acyclovir should be considered in the treatment of disseminated HSV infection in pregnancy.
===================================================================
13.) Acyclovir for disseminated herpes simplex virus in pregnancy. A case report.
===================================================================
J Reprod Med 1994 Apr;39(4):311-7
Greenspoon JS, Wilcox JG, McHutchison LB, Rosen DJ
Department of Obstetrics and Gynecology, Cedars-Sinai Medical Center, Los Angeles, CA 90048.
Seventeen cases of disseminated herpes simplex virus (HSV) infection have occurred during pregnancy. Acyclovir therapy was associated with prolongation of the time from admission until spontaneous rupture of the membranes or delivery and an improved maternal outcome. This life-threatening condition has a typical presentation, which includes a nonspecific viral prodrome.
During pregnancy, fever and anicteric hepatitis unresponsive to empiric antibiotics should prompt an evaluation for disseminated herpes simplex. Pharyngitis or skin lesions with a positive herpes simplex culture are common, specific signs associated with dissemination. The fever resolves within 48 hours in response to acyclovir therapy. One case of maternal disseminated HSV occurred at 22 weeks' gestation and resolved with acyclovir therapy; a healthy neonate was delivered vaginally at term.
===================================================================
14.) Acyclovir for the prevention and treatment of varicella zoster in children, adolescents and pregnancy.
===================================================================
J Paediatr Child Health 1996 Jun;32(3):211-7
Kesson AM, Grimwood K, Burgess MA, Ferson MJ, Gilbert GL, Hogg G, Isaacs D, Kakakios A, McIntyre P
Australasian Society for infectious Diseases, Sydney, New South Wales, Australia.
Varicella causes a mild, self-limiting childhood disease that may reactivate years later as shingles. In immunocompromised patients with altered cell mediated immunity, and rarely in healthy individuals, varicella results in a life-threatening infection.
The antiviral drug, acyclovir, substantially reduces the mortality and risk of severe disease in these groups of patients. Early commencement of acyclovir is recommended for children with both varicella and altered cell mediated immunity, newborns during the first 2 weeks of life, preterm infants in the neonatal nursery, and severe varicella or shingles (including ocular zoster) in any patient, as well as during pregnancy.
Acyclovir may be considered in children with serious cardiopulmonary disease or chronic skin disorders where varicella may exacerbate the underlying disease or increase the risk of secondary bacterial sepsis. Acyclovir, however, is not recommended for healthy individuals without severe disease, as a prophylactic agent against varicella, for asthmatics receiving aerosolized or low-dose oral steroids and/or as treatment of the post-varicella syndromes. When acyclovir is prescribed it should be given intravenously to those with severe disease, those at risk of dissemination and in children younger than 2 years of age.
===================================================================
15.) Treatment with acyclovir of varicella pneumonia in pregnancy.
===================================================================
Chest 1991 Apr;99(4):1045-7
Broussard RC, Payne DK, George RB
Department of Medicine, Louisiana State University School of Medicine, Shreveport.
Varicella pneumonia during pregnancy carries a significant mortality for both mother and fetus. The antiviral drug, acyclovir, appears to have decreased mortality in reported cases. We present a case report and review of the literature summarizing the experience to date with acyclovir in the treatment of varicella pneumonia during pregnancy.
===================================================================
16.) Varicella pneumonia during pregnancy. Treatment of two cases with acyclovir.
===================================================================
Am J Perinatol 1988 Jan;5(1):16-8
Eder SE, Apuzzio JJ, Weiss G
Department of Obstetrics and Gynecology, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark 07103.
Pneumonia is a rare but serious complication of varicella during pregnancy. Maternal mortality has been reported to be 41% with fetal and neonatal mortality at 65%. Treatment has included respiratory support and prophylactic antibiotics.
Acyclovir has been prescribed with the intent to decrease the impact of the infection. It was added to the treatment protocol of two cases of varicella pneumonia in pregnancy. Despite the high maternal and perinatal mortality both pairs of patients and infants survived. Acyclovir did not appear to adversely influence the fetus, and may have contributed to the survival of mother and child.
===================================================================
17.) Use of acyclovir for varicella pneumonia during pregnancy.
===================================================================
Obstet Gynecol 1991 Dec;78(6):1112-6
Smego RA Jr, Asperilla MO
Section of Infectious Diseases, West Virginia University Health Sciences Center, Morgantown.
Twenty-one cases (five new and 16 literature) of varicella pneumonia of pregnancy were retrospectively reviewed to evaluate the benefits and risks of intravenous acyclovir on maternal and fetal outcomes. All women were in their second (12 cases) or third (nine cases) trimester. Mean gestational ages at the onset of pneumonia and time of delivery were 27 and 36 weeks, respectively. Twelve patients required mechanical ventilation. The mean duration of treatment was 7 days.
No definite adverse drug effects were noted. Three women (14%) died of uncontrolled infection or complications. Two infants died (whose mothers also died): One was stillborn at 34 weeks' gestation, and the other died from prematurity shortly after birth at 26 weeks. No child was born with features of congenital varicella syndrome, and none developed active perinatal varicella infection.
Onset of pneumonia during the third trimester was a risk factor associated with fatal maternal outcome. Intravenous acyclovir may reduce maternal morbidity and mortality associated with varicella pneumonia occurring during pregnancy, and appears to be safe for the developing fetus when given during the latter trimesters.
===================================================================
18.) Varicella during pregnancy. Maternal and fetal effects.
===================================================================
West J Med 1995 Nov;163(5):446-50
Katz VL, Kuller JA, McMahon MJ, Warren MA, Wells SR
Dept of Obstetrics and Gynecology, University of North Carolina Hospital, Chapel Hill 27599-7570, USA.
To determine the characteristics of maternal varicella at our institution, we reviewed all cases of primary varicella in pregnancy. Using a perinatal database that summarizes all obstetric admissions, we reviewed the medical records of women with varicella infections during pregnancy.
Over a 5 1/2-year period, 31 pregnancies were affected by varicella infection among 11,753 deliveries. The mean age of those patients was 19.6 years, significantly different from our overall population of 25.3 years (P < .05). The racial composition of 35% Hispanic, 35% white, and 29% African American was different from that of our general population of 55% white, 38% African American, and 6% Hispanic (P = .023). The mean gestational age of the eruption of vesicles was 25 weeks.
Of the 31 women, 7 had preterm labor within a week of their varicella, 3 delivered prematurely, and 3 infants had a birth weight of less than 2,700 grams. Respiratory symptoms developed in 6 women, and pneumonia developed in 4, 2 of whom required ventilatory support, 1 for 5 days, the other for 49 days. Eight women received acyclovir during gestation, and none suffered sequelae. In all, 6 infants had lesions and anomalies noted at birth, 5 possibly associated with varicella.
Varicella infection is associated with a greater-than-expected level of both maternal and fetal morbidity. The fetal disease may occur due to maternal infection at any gestation and is most likely a spectrum of complications. The maternal disease appears to be worse in the latter half of pregnancy. Programs of prevention through vaccination must account for a possibly decreased level of immunity in different populations.
===================================================================
19.) Perinatal outcome of pregnancies complicated with varicella infection during the first 20 weeks of gestation.
===================================================================
Am J Perinatol 1997 Aug;14(7):411-4
Figueroa-Damian R, Arredondo-Garcia JL
Infectious Diseases Department, National Institute of Perinatology, Mexico, D.F., Mexico.
Varicella-Zoster (V-Z) virus infection during pregnancy is uncommon. Nevertheless, it has importance due to the risk of vertical transmission of the infection and also because of a higher morbidity rate among pregnant women. The cases of varicella infection that occur in the first and second trimesters of pregnancy are occasionally associated to the development of congenital varicella syndrome.
We studied 22 women whose pregnancy was complicated with varicella during the first 20 weeks of gestation. The average age of these patients was 20 +/- 3.6 years with a range of 16 to 20 years. None of the patients presented complications due to the V-Z virus infection. Two pregnancies finalized in preterm labor. None of the newborns had congenital anormalies; one presented microcephaly, and another low birth weight.
There was no significant difference between the infants of women with varicella and those of the controls in birth weight, size, and head circumference. We concluded that varicella infection during the first 20 weeks of gestation was not associated with serious maternal morbidity, and has low repercussion in the pregnancy outcome and the fetus.
===================================================================
20.) Varicella and pregnancy.
===================================================================
Eur J Obstet Gynecol Reprod Biol 1996 Jun;66(2):119-23
Dufour P, de Bievre P, Vinatier D, Tordjeman N, Da Lage B, Vanhove J, Monnier JC
Service of Gynecology-Obstetrics, Pr. J.C. Monnier, CHRU de Lille, France.
OBJECTIVE: To appreciate the risk of embryo-foetopathy in case of maternal varicella occurring before 20 weeks of gestation, as well as the maternal complication risk (notably pulmonary) in case of maternal varicella occurring the third trimester of pregnancy.
METHOD: Over the period from January 1987 to February 1995, 20 patients were managed for maternal varicella confirmed during the pregnancy. From these observations, the authors, by studying the literature, attempt to better specify the real fetal and/or maternal complication risk in case of maternal varicella.
RESULTS: In their personal series of 20 cases, including 17 before 20 weeks of gestation, the authors have noted no embryo-foetopathy. Similarly, no maternal complication (notably pulmonary complication), has been found. Careful study of the literature allows to specify some points. In case of varicella before 20 weeks, one observes an identical frequency of spontaneous abortions, as compared to the general population and a moderated increase of the frequency of premature delivery.
The risk of congenital varicella syndrome reaches about 1.3%. Finally the risk of neonatal varicella consists in a maternal infection which occurs during the perinatal period and which is source of a high perinatal morbidity. The prenatal diagnosis is based essentially and currently, on the amniocentesis with viral research by polymerase chain reaction (PCR) in the amniotic fluid, completed by a ultrasound supervision.
CONCLUSION: The occurrence of maternal varicella during the pregnancy is rare (0.7/1000) because more than 90% of women are immunized. The risk of congenital varicella syndrome is limited to the 20 first weeks and seems very weak, authorizing therapists to reassure patients presenting a varicella during their pregnancy. Nevertheless, the risk of pulmonary complications for the mother, in case of varicella during the third trimester, does exist and requires appropriated treatment.
===================================================================
21.) Intrauterine infection with varicella-zoster virus after maternal varicella.
===================================================================
N Engl J Med 1986 Jun 12;314(24):1542-6
Paryani SG, Arvin AM
We investigated the consequences of maternal infection with varicella-zoster virus in a prospective study of 43 pregnancies complicated by varicella and 14 pregnancies complicated by herpes zoster. Nine of 43 pregnant women with varicella had associated morbidity--pneumonia (4 women), death (1), premature labor (4 of 42), premature delivery (2 of 42), and herpes zoster (1).
Intrauterine varicella infection was identified on the basis of clinical evidence (anomalies characteristic of the congenital varicella syndrome, acute varicella at birth, or herpes zoster in infancy) or immunologic evidence (IgM antibody to varicella-zoster in the neonatal period, persistent IgG antibody to varicella-zoster at one to two years of age, or in vitro lymphocyte proliferation in response to varicella-zoster virus antigen).
The congenital varicella syndrome occurred in 1 of 11 infants of women with first-trimester varicella. Immunologic evidence of intrauterine varicella infection was present in 7 of 33 infants tested; 4 of these infants were asymptomatic. According to clinical or immunologic criteria, 8 of 33 infants had evidence of intrauterine varicella infection. These observations show that varicella during pregnancy was associated with maternal morbidity and evidence of fetal infection, but that herpes zoster was not.
===================================================================
22.) [Varicella in pregnancy after the 20th week of amenorrhea].
===================================================================
J Gynecol Obstet Biol Reprod (Paris) 1992;21(8):935-42
Pierre JC, Senneville E, Ajana F, Santre C, Chidiac C, Mouton Y
Centre Hospitalier de Tourcoing, Services des Maladies Infectieuses et du Voyageur.
We report five cases of varicella pneumonia among ten otherwise healthy pregnant women who were admitted in our hospital between 1986 and 1991 with chickenpox. The precise frequency of this rare complication is not well known actually but analysis of the literature shows that the mortality rate is about 20%. Beside the problem of the fetal varicella syndrome, the other complication is the severe varicella of the neonate which can appear when varicella occurs in the mother within 5 days before, and 2 days after delivery.
When primary varicella infection occurs during pregnancy clinical examination must be repeated for a week after occurring of the exanthema to find elements of severity significance. Acyclovir is the drug of choice (10 to 15 mg/kg every 8 hours) for 7 days when pneumonia is present. Varicella-zoster immunoglobulin is useful for prophylaxis and for neonates with high risk of severe varicella.
===================================================================
23.) Varicella in pregnancy.
===================================================================
Semin Perinatol 1998 Aug;22(4):339-46
Chapman SJ
Center for Women's Medicine, Division of Maternal-Fetal Medicine, Greenville Hospital System, SC 29605, USA.
Varicella-zoster virus may cause serious infection, particularly pneumonia, in adult women. Women of child-bearing age should be questioned about immunity to varicella preconceptually, and offered serological testing, and VARIVAX vaccine if indicated. All pregnant patients should be questioned about immunity to varicella during their first prenatal appointment.
Susceptible patients should be counseled to avoid contact with individuals who have chickenpox. If exposure occurs, VZIG should be administered within 96 hours in an attempt to prevent maternal infection. Varicella embryopathy may occur as a result of maternal infection particularly in the first half of pregnancy with an incidence of 1% to 2%.
Varicella of the newborn is a life-threatening illness that may occur when a newborn is delivered within 5 days of the onset of maternal illness or after postdelivery exposure to varicella. Susceptible neonates should receive VZIG. Acyclovir is active against the varicella-zoster virus, and treatment is indicated in seriously ill adults and neonates.
===================================================================
24.) [Chickenpox and pregnancy. Perinatal aspects and prevention].
===================================================================
Arch Fr Pediatr 1987 May;44(5):339-42
Haddad J, Roth S, Simeoni U, Gut JP, Messer J, Willard D
Five neonates born to women who had had varicella late in pregnancy or in the post-partum were admitted to our unit during the last year. In utero transmission of varicella-zoster virus occurred in 2 cases. One of them had no clinical eruption but specific IgM at a titer of 1/200. The mother presented with varicella 15 days before delivery. The other developed severe neonatal congenital varicella (with disseminated eruption, pneumonia and seizures). She was treated by Aciclovir (15 mg/kg/8 h).
The mother presented with chickenpox 24 hours after birth. Varicella occurring in a pregnant woman from 4 days before to 2 days after delivery is dangerous because the baby will lack maternal antibodies. It may develop severe neonatal varicella (mortality: 20-30%). A neonate in critical condition was successfully given a prophylactic treatment by Aciclovir IV (15 mg/kg/8 h for 5 days) and varicella-zona immunoglobulins (2 ml on days 1, 2, 3). This approach may be the best treatment for babies at risk for severe neonatal varicella.
===================================================================
25.) Use of acyclovir for varicella pneumonia during pregnancy.
===================================================================
Obstet Gynecol 1991 Dec;78(6):1112-6
Smego RA Jr, Asperilla MO
Section of Infectious Diseases, West Virginia University Health Sciences Center, Morgantown.
Twenty-one cases (five new and 16 literature) of varicella pneumonia of pregnancy were retrospectively reviewed to evaluate the benefits and risks of intravenous acyclovir on maternal and fetal outcomes. All women were in their second (12 cases) or third (nine cases) trimester. Mean gestational ages at the onset of pneumonia and time of delivery were 27 and 36 weeks, respectively.
Twelve patients required mechanical ventilation. The mean duration of treatment was 7 days. No definite adverse drug effects were noted. Three women (14%) died of uncontrolled infection or complications. Two infants died (whose mothers also died): One was stillborn at 34 weeks' gestation, and the other died from prematurity shortly after birth at 26 weeks.
No child was born with features of congenital varicella syndrome, and none developed active perinatal varicella infection. Onset of pneumonia during the third trimester was a risk factor associated with fatal maternal outcome. Intravenous acyclovir may reduce maternal morbidity and mortality associated with varicella pneumonia occurring during pregnancy, and appears to be safe for the developing fetus when given during the latter trimesters.
===================================================================
26.) Fatal disseminated herpes simplex in pregnancy with maternal and neonatal death.
===================================================================
South Med J 1996 Jul;89(7):732-4
Gelven PL, Gruber KK, Swiger FK, Cina SJ, Harley RA
Department of Pathology and Laboratory Medicine, Medical University of South Carolina , Charleston 29425, USA.
Disseminated herpes is rare in the adult and usually occurs in the immunocompromised. Twenty-one cases have been reported in which healthy women contracted life-threatening disseminated herpes simplex virus (HSV) infections in the third trimester of pregnancy. Most of these patients had nonspecific symptoms, and many did not have mucocutaneous lesions.
On physical examination, they were usually febrile and anicteric and had markedly elevated aminotransferase values, without a corresponding elevation in bilirubin level. In our review of the literature, we found that prompt acyclovir therapy resulted in 100% survival.
Those patients not receiving treatment or treated late in the terminal stages of their disease had a 63% mortality rate. We report a case of maternal disseminated HSV with subsequent maternal death at an estimated 31 weeks' gestation in which the diagnosis was made at the time of necropsy. The infant was started on acyclovir therapy but died of disseminated HSV.
===================================================================
27.) Connatal herpes zoster.
===================================================================
Cutis 1996 Sep;58(3):231-4
Querol I, Bueno M, Cebrian A, Gonzalez-Echeverria FJ
Department of Dermatology, Hospital Reina Sofia de Tudela, Spain.
We describe a case of connatal herpes zoster present in a newborn girl whose mother had been exposed to varicella infection during the seventh month of pregnancy. A few minutes after delivery, the newborn was examined for an erythematous maculopapular rash with clear grouped vesicles involving the right L2-L4 dermatome.
She was given varicella zoster immunoglobulin and oral and topical acyclovir, and all the skin lesions were completely healed eight days later. This report emphasizes one aspect of the relationship between maternal exposure to varicella zoster virus infection and the occurrence of connatal shingles, the benign course of the disease in this case, and the favorable response to acyclovir therapy in neonates.
===================================================================
28.) Pregnancy outcomes following systemic prenatal acyclovir exposure--June 1, 1984-June 30, 1993.
===================================================================
MMWR Morb Mortal Wkly Rep 1993 Oct 22;42(41):806-9
Herpes infections are common among women of reproductive age (i.e., aged 15-44 years). Acyclovir (Zovirax), an antiviral drug effective in the treatment of herpes simplex infection, was approved by the Food and Drug Administration (FDA) in 1984. Since its approval, the effects of acyclovir on human pregnancies have not been determined.
However, inadvertent pregnancy exposures to acyclovir were expected to occur among women in whom treatment had been indicated for preexisting herpes simplex infections. Some physicians have reported intentional use of acyclovir during pregnancy for treatment of life-threatening herpes simplex infection.
To assess the outcomes of pregnancies exposed to acyclovir, the Acyclovir in Pregnancy Registry was established on June 1, 1984, by the manufacturer, in collaboration with CDC. This report summarizes data on pregnancies reported to the registry through June 30, 1993.
===================================================================
29.) Severe pneumonia in pregnancy three months after resolution of cutaneous zoster.
===================================================================
Infection 1994 May-Jun;22(3):216-8
Moling O, Mayr O, Gottardi H, Mian P, Zanon P, Oberkofler F, Gramegna M, Colucci G
Sektion fur Infektionskrankheiten, Medizinische Abt. I, Allgemeines Regionalkrankenhaus Bozen, Italy.
A 22 weeks pregnant women was affected by a life-threatening pneumonia and a paresis of the proximal muscles with cerebrospinal fluid pleocytosis. Her past medical history had been unremarkable except for recurrent episodes of paraumbilical herpes zoster. The clinical findings suggested a dissemination of varicella-zoster virus without skin lesions. Acyclovir was added to the therapy, and the clinical picture began to improve. Varicella-zoster virus DNA was detected in placental tissue by DNA-hybridisation analysis.
===================================================================
30.) Disseminated herpes zoster in a pregnant woman positive for human immunodeficiency virus.
===================================================================
Am J Perinatol 1993 Nov;10(6):463-4
Petrozza JC, Monga M, Oshiro BT, Graham JM, Blanco JD
Department of Obstetrics, Gynecology and Reproductive Sciences, Lyndon B. Johnson General Hospital, University of Texas Health Science Center, Houston 77026.
We report a case of disseminated herpes zoster in a pregnant patient positive for the human immunodeficiency virus (HIV). Disseminated zoster was the first manifestation of HIV infection in this patient. In HIV-positive patients, zoster may be complicated by cutaneous dissemination, visceral involvement, and death. Intravenous acyclovir may prevent serious sequelae in both mother and fetus.
===================================================================
31.) Early-second-trimester use of acyclovir in treating herpes zoster in a
bone marrow transplant patient. A case report.
===================================================================
J Reprod Med 1992 Mar;37(3):280-2
Horowitz GM, Hankins GD
Department of Obstetrics and Gynecology, Wilford Hall United States Air Force Medical Center, Lackland Air Force Base, Texas 78236-5300.
Bone marrow transplantation from a human leukocyte antigen (HLA)-identical sibling for treatment of severe aplastic anemia among women of reproductive age is becoming more common. Successful pregnancy has been reported to occur in several such patients.
A woman delivered a healthy, term, female infant 18 months after a transplant from her HLA-identical sister. Her pregnancy was complicated by disseminated herpes zoster, treated with intravenous acyclovir at 14 weeks' gestation, before the diagnosis of pregnancy. While there have been several case reports involving the use of acyclovir in the third trimester, primarily in the treatment of varicella infections, there have been no previous reports of such an early utilization of this antiviral drug.
===================================================================
32.) [Acyclovir and pregnancy: current aspects].
===================================================================
J Gynecol Obstet Biol Reprod (Paris) 1989;18(5):679-83
Haddad J, Messer J, Willard D, Ritter J
Service de Neonatologie, CHU Hautepierre, Strasbourg.
Acyclovir (ACV), an antiviral nucleoside analog, is active against Herpes simplex viruses (HSV1, HSV2) and varicella virus (VZV). These viruses seems to be prejudicial to the pregnant woman and to the fetus. Yet, ACV is not recommended for use in pregnancy.
However in certain cases, this drug has been used. We review in this paper, the pharmacokinetics and transplacental passage of ACV, indications, and whether the benefits of the administration of ACV in pregnancy outweigh the theoretical risks. Peak and trough plasma concentrations of ACV in pregnant women seem to be lower as compared to those of non-pregnant adults but effective.
This drug crosses the placenta. Levels of ACV in cord blood ranged from 0.5 to 3 mumol/l. In as much as in vitro inhibitory doses 50 (ID 50) for HSV1, HSV2 and VZV ranged from 0.1 to 3 mumol/l, it is quite likely that levels noted above may be effective for in utero inhibition of viral replication.
No adverse effects were noted in newborn exposed in utero to ACV. But one must be careful about the direct effects of this drug on nucleic acid metabolism despite encouraging results on animal fetuses.
Based on these findings and from our experience, ACV can be administered in pregnancy in two particular situations: in cases of maternal severe viral infections and in order to inhibit in utero VZV replication. Doses required for pregnant women range from 5 to 15 mg/kg/8 hours given intravenously, and 200 mg of oral Acyclovir 5 times daily.
===================================================================
33.) [A case of delayed cerebral infarction occurring in puerperium preceded by herpes zoster ophthalmicus in late pregnancy].
===================================================================
No To Shinkei 1999 Jun;51(6):529-33
Hoshino S, Hayashi A, Yoshizawa T, Tamaoka A, Shoji S
Department of Neurology, University of Tsukuba, Japan.
Delayed central neurological symptoms following herpes zoster ophthalmicus (HZO) such as "herpes zoster ophthalmicus and delayed contralateral hemiparesis" are considered to be due to ipsilateral intracranial vasculopathy.
We experienced a rare case with cerebral infarction occurred in puerperium following HZO in late pregnancy. A healthy 30-year-old woman had left HZO at weeks 35 of gestation. She was given acyclovir (ACV) for external use and improved with small pigmentation on the left eye-lid. Seven weeks after the onset of HZO, she suddenly developed aphasia and right hemiparesis. Cerebral angiogram showed narrowing on M 1 segment of the ipsilateral middle cerebral artery.
The occlusion was seen on peripheral portion of the angular artery on the same side. In cerebrospinal fluid (CSF), cell count was slightly elevated, but concentration of protein and sugar were normal. Varicella-zoster titer was increased in both serum and CSF.
She was treated with intravenous ACV (1500 mg/day) for 10 days. On the next day after the treatment, the cell count was normalized and on 18th day, varicella-zoster titer was decreased in CSF. Higher brain function improved and no relapses occurred. This is a first case of delayed cerebral infarction occurring in puerperium preceded by herpes zoster ophthalmicus in late pregnancy, as far as we searched. We should treat carefully pregnant or lactating patients with HZO, considering delayed cerebral infarction.
===================================================================
34.) Congenital varicella-zoster virus infection and Barrett's esophagus.
===================================================================
J Infect Dis 1998 Aug;178(2):539-43
Ussery XT, Annunziato P, Gershon AA, Reid BS, Lungu O, Langston C, Silverstein S, Lee KK, Baker CJ
Department of Pediatrics, Baylor College of Medicine, Houston, Texas 77030, USA.
Congenital varicella syndrome is a rare complication of varicella-zoster virus (VZV) infection during pregnancy. An infant was exposed to VZV at 18.5 weeks of gestation and had eye and skin abnormalities at birth and persistent feeding difficulties, prompting esophageal biopsies at 12 days and 20 and 20.5 months of age.
Esophageal tissues demonstrated specialized intestinal metaplasia (Barrett's esophagus). VZV DNA (in situ hybridization) and proteins (immunohistochemistry and polymerase chain reaction) were found in esophageal epithelial cells adjacent to the Barrett's lesion. Immediate-early 63 protein (IE63) of VZV was demonstrated in the day 12 specimen, and IE62 and the late VZV glycoprotein E (gE) were found in the 20-month specimen.
Clinical and endoscopic improvement followed fundoplication and acyclovir therapy, but VZV DNA and IE62 persisted in esophageal tissue. These findings associate VZV with specialized intestinal metaplasia within the esophagus and suggest a novel site for either latent or active VZV infection.
===================================================================
35.) Antibodies to varicella zoster virus in the cerebrospinal fluid of neonates with seizures.
===================================================================
Arch Dis Child Fetal Neonatal Ed 1998 Jan;78(1):F57-61
Mustonen K, Mustakangas P, Smeds M, Mannonen L, Uotila L, Vaheri A, Koskiniemi M
Department of Neuropediatrics, North Karelia Central Hospital, Ioensu, Finland.
Four neonates with convulsions had IgG antibodies in their cerebrospinal fluid (CSF) to varicella zoster virus (VZV). These antibodies were found in the sera of two of these patients after the age of 6 months. Antibodies to 16 different microbes were studied from the serum and CSF of 201 neonates with neurological problems.
The presence of DNA specific to HSV-1, HSV-2, and VZV in the CSF was also investigated using the polymerase chain reaction (PCR). Antibodies to VZV were detected in the CSF of four neonates. Antibody indices suggested production of VZV specific antibodies in the central nervous system. These findings suggest that intrathecal production of antibodies to VZV can appear in neonates with neurological problems, which suggests that intrauterine VZV infection can be acquired without cutaneous symptoms in the mother.
===================================================================
36.) ACYCLOVIR (Systemic)
===================================================================
INN: Aciclovir
VA CLASSIFICATION (Primary/Secondary;AM800
Commonly used brand name(s):
Avirax;
Zovirax.
Note: For a listing of dosage forms and brand names by country availability, see Dosage Forms section(s).
Category
Antiviral (systemic).
Indications
Note: Bracketed information in the Indications section refers to uses that are not included in U.S. product labeling.
Accepted
Herpes genitalis (treatment;Oral acyclovir is indicated in the treatment of initial episodes and management of recurrent, severe herpes genitalis infections in immunocompromised and nonimmunocompromised patients. Parenteral acyclovir is indicated in the treatment of severe initial herpes genitalis infections in immunocompromised and nonimmunocompromised patients, and in patients who are unable to take (or absorb) oral acyclovir.2,63
Herpes genitalis (prophylaxis;Oral acyclovir is indicated in the prophylaxis of frequently recurrent (³ 6 episodes per year) herpes genitalis infections in immunocompromised and nonimmunocompromised patients.2,14,26,27
Herpes simplex (treatment;Parenteral [and oral] acyclovir are indicated in the treatment of initial and recurrent mucocutaneous herpes simplex (HSV-1 and HSV-2) infections in immunocompromised patients.1,50,59,61
[Herpes simplex (prophylaxis)]*:Parenteral and oral acyclovir are used in the prophylaxis of herpes simplex virus (HSV) infections in patients who are immunocompromised, including transplant patients receiving immunosuppressant therapy, human immunodeficiency virus (HIV)-infected patients, and patients receiving chemotherapy.14,23,24,25,50,65,93
Herpes simplex encephalitis (treatment)*:Parenteral acyclovir is indicated in the treatment of herpes simplex encephalitis.17,18,44,46,67
Herpes zoster (treatment;Oral acyclovir is indicated in the treatment of herpes zoster infections (shingles) caused by varicella-zoster virus (VZV) in any adult patient with herpes zoster. Therapy is most effective when started within 48 hours of the onset of rash.44,45,50,66 Parenteral acyclovir is indicated in the treatment of herpes zoster infections (shingles) caused by VZV in immunocompromised patients and disseminated herpes zoster in nonimmunocompromised patients.15,22,67
[Herpes zoster (prophylaxis)]*:Oral acyclovir is used in the prophylaxis of herpes zoster infections (shingles) caused by VZV, after an initial period of parenteral acyclovir, in any immunocompromised patient, including transplant patients receiving immunosuppressant therapy, HIV-infected patients, and patients receiving chemotherapy.50,55
Herpes zoster ophthalmicus (treatment;Oral and parenteral acyclovir are indicated in the treatment of herpes zoster ophthalmicus.73,74,75,95
[Herpes simplex virus, disseminated neonatal infection (treatment)]*:Parenteral acyclovir is used in the treatment of disseminated HSV in neonates.14,61,63
Varicella (treatment;Oral acyclovir is indicated in the treatment of varicella infections (chickenpox) in nonimmunocompromised patients when started within 24 hours of the onset of a typical chickenpox rash.72,88,90,92[ Parenteral acyclovir is used in the treatment of varicella infections (chickenpox) caused by VZV in immunocompromised patients.]21
Although acyclovir is indicated for the treatment of varicella infections in nonimmunocompromised patients, the American Academy of Pediatrics does not recommend its use for the treatment of uncomplicated chickenpox in healthy children. It is recommended for certain groups at increased risk of severe varicella or its complications, such as otherwise healthy, nonpregnant persons 13 years of age or older; children older than 12 months of age with a chronic cutaneous or pulmonary disorder; and children receiving short, intermittent or aerosolized courses of corticosteroids. If possible, steroids should be discontinued after known exposure to varicella.94
Resistance of HSV and VZV to acyclovir has been reported to develop with prolonged treatment or repeated therapy in severely immunocompromised patients. Resistance may occasionally develop as quickly as within a few weeks. If lesions due to herpes simplex virus fail to respond to acyclovir therapy, especially with continued viral shedding, viral isolates should be tested for susceptibility to acyclovir.2,10,63
Unaccepted
Oral acyclovir is not indicated in the suppression of recurrent herpes genitalis in patients with infrequent recurrences.2
*Not included in Canadian product labeling.
*Not included in Canadian product labeling.
Pharmacology/Pharmacokinetics
Physicochemical characteristics:
Molecular weight:
5Acyclovir: 225.21
Acyclovir sodium: 247.19
pH:
Reconstituted acyclovir (50 mg per mL): Approximately 1149.
Mechanism of action/Effect:
Acyclovir is converted to acyclovir monophosphate, a nucleotide, by the viral thymidine kinases of herpes simplex virus (HSV) and varicella-zoster virus (VZV). Acyclovir monophosphate is converted to the diphosphate by cellular guanylate kinase and to the triphosphate by a number of cellular enzymes. Acyclovir triphosphate interferes with HSV and VZV DNA polymerase and inhibits viral DNA replication. The triphosphate can be incorporated into growing chains of DNA by viral DNA polymerase, resulting in termination of the DNA chain.1,2,44
Absorption:
Oral:Bioavailability 20% (range, 15 to 30%).54 Poorly absorbed from the gastrointestinal tract. Not significantly affected by food.2,4,44
Distribution:
Widely distributed to tissues and body fluids, including brain, kidneys, lungs, liver, aqueous humor, tears, intestines, muscle, spleen, breast milk, uterus, vaginal mucosa, vaginal secretions, semen, amniotic fluid, cerebrospinal fluid (CSF), and herpetic vesicular fluid. Highest concentrations are found in kidneys, liver, and intestines. CSF concentrations are approximately 50% of plasma concentrations. Crosses the placenta, also.1,4,50,76
VolD (steady state;
Adults: Approximately 48 liters (L) per square meter of body surface (m2)
(range, 37 to 57 L per m2).11,53
Children and adolescents (1 to 18 years old): Approximately 45 L per m2.11
Neonates (0 to 3 months old): Approximately 28 L per m2 (range, 24 to 30 L
per m2).11,52
End-stage renal disease: Approximately 41 L per m2.11
Protein binding:
Low (9 to 33%).95
Biotransformation:
Hepatic; only major metabolite found in urine is 9-carboxymethoxymethylguanine, which accounts for approximately 9 to 14% of the dose. This metabolite has no known antiviral activity.4,47
Half-life:
Intravenous:
Adults: Approximately 2.5 hours.54
Children (1 to 18 years old): Approximately 2.6 hours.54
Neonates (0 to 3 months old): Approximately 4 hours.48,50
Renal impairment (adults)1,50:
Creatinine Clearance Half-life
(mL/min)/(mL/sec) (hr)
>80/1.33 2.5
50-80/0.83-1.33 3.0
15-50/0.25-0.83 3.5
Anuric 19.5
During hemodialysis 5.7
Continuous ambulatory 14-18
peritoneal dialysis
Oral:
3.3 hours.54
Time to peak serum concentration
Intravenous:End of infusion (approximately 1 hour).
Oral:1.7 hours.48
Mean peak serum concentration (steady-state)
Oral:Adults:
200 mg every 4 hours:
0.6 mcg/mL (2.5 micromoles/L).2
400 mg every 4 hours:
1.2 mcg/mL (5.3 micromoles/L).2
800 mg every 4 hours:
1.6 mcg/mL (6.9 micromoles/L).14
Intravenous:
Adults:
5 mg per kg (over 1 hour) every 8 hours:9.8 mcg/mL (43.5 micromoles/L).
10 mg per kg (over 1 hour) every 8 hours:22.9 mcg/mL (101.7 micromoles/L).
Children (1 to 18 years old)53:
250 mg per m2 (over 1 hour) every 8 hours:10.3 mcg/mL (45.8 micromoles/L).
500 mg per m2 (over 1 hour) every 8 hours:20.7 mcg/mL (91.9 micromoles/L).
Neonates (0 to 3 months old)52:
5 mg per kg (over 1 hour) every 8 hours:6.8 mcg/mL (30 micromoles/L).
10 mg per kg (over 1 hour) every 8 hours:13.8 mcg/mL (61.2 micromoles/L).
Elimination:
Renal:
Excreted by both glomerular filtration and tubular secretion.67
Oral: Approximately 14% of total dose excreted unchanged in urine.91,95
Intravenous: Approximately 45 to 79% excreted unchanged in urine.95
Fecal:
Insignificant amounts (<2%).1,11
Lungs:
Trace amounts in exhaled CO2.1,11
Dialysis:
Hemodialysis: A single 6-hour period of hemodialysis reduces plasma acyclovir concentrations by approximately 60%.1
Peritoneal dialysis: Peritoneal dialysis does not substantially alter acyclovir clearance.51
Precautions to Consider
Cross-sensitivity and/or related problems
Patients allergic to ganciclovir may also be allergic to acyclovir because of the chemical similarity of the two medications.
Carcinogenicity
Life-time bioassays in rats and mice given daily doses of 50, 150, and 450 mg per kg of body weight (mg/kg) by gavage have not shown any evidence of carcinogenicity. However, in vitrocell transformation assays have given conflicting results, being positive at the highest dose used in one system. The resulting morphologically transformed cells induced tumors when inoculated into immunosuppressed, syngeneic, weanling mice, although results were negative in another transformation assay.1
Mutagenicity
Oral acyclovir has been shown to be mutagenic at high concentrations in some acute animal studies. However, no chromosomal damage was noted at maximum tolerated parenteral doses (100 mg/kg) in rats or Chinese hamsters. Higher doses (500 and 1000 mg/kg) were clastogenic in Chinese hamsters. No problems were reported in dominant lethal studies in mice. Also, there was no evidence of mutagenicity in 9 out of 11 microbial and mammalian cell assays. In 2 of the mammalian cell assays, a positive response for mutagenicity and chromosomal damage was noted, but only at concentrations at least 25 times the usual plasma concentrations achieved in humans.1
Pregnancy/Reproduction
Fertility:Impairment of spermatogenesis, sperm motility, or morphology has not been documented in humans.77 However, high doses of parenteral acyclovir have caused testicular atrophy in rats and dogs. Some evidence of sperm production recovery was evident 30 days post-dose. Studies in mice given oral doses of up to 450 mg/kg per day have shown that acyclovir does not impair fertility or reproduction. Studies in female rabbits given acyclovir subcutaneously subsequent to mating have shown a significant decrease in implantation efficiency, but no decrease in litter size at doses of 50 mg/kg per day.1
Pregnancy:Acyclovir crosses the placenta.4 Acyclovir has been used in all stages of pregnancy, most commonly in the third trimester. No adverse fetal effects have been reported.36,81 One small, controlled study found that pre-partum treatment of women with recurrent genital herpes helped prevent symptomatic recurrences and viral shedding at the time of delivery, reducing the risk of the infant being exposed to the virus.82Adequate and well-controlled studies in humans have not been done.
Studies in mice given oral doses of 450 mg/kg per day and studies in rats and rabbits given subcutaneous doses of 50 mg/kg per day have shown that acyclovir does not cause adverse effects in the fetus.1
FDA Pregnancy Category C.
Breast-feeding
Acyclovir passes into breast milk at concentrations from 0.6 to 4.1 times the corresponding plasma concentration. A very small amount of acyclovir has been measured in one nursing infant's urine; no toxicity was observed.56,57
Pediatrics
Limited data are available about the use of oral acyclovir in children younger than 2 years of age. However, no unusual toxicity or pediatrics-specific problems have been observed in studies done in children using doses of up to 3000 mg per square meter of body surface area (mg/m2) per day and 80 mg/kg per day.55,68,69,70,71,72Intravenous acyclovir should be used with greater caution in neonates due to their age-related decrease in clearance.51 The half-life and clearance of intravenous acyclovir in children older than 1 year of age is similar to that seen in adults with normal renal function.48
Geriatrics
Studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of acyclovir in the elderly. However, elderly patients are more likely to have an age-related decrease in renal function, which may require an adjustment of acyclovir dosage or dosing interval.
Drug interactions and/or related problems
The following drug interactions and/or related problems have been selected on the basis of their potential clinical significance (possible mechanism in parentheses where appropriate;not necessarily inclusive (>> = major clinical significance):
Note: Combinations containing any of the following medications, depending on the amount present, may also interact with this medication.
>> Nephrotoxic medications, other (See Appendix II)1:(concurrent use with intravenous acyclovir may increase the potential for nephrotoxicity, especially in the presence of renal function impairment)
Probenecid1,2,54,80:(may decrease renal tubular secretion of intravenous acyclovir when used concurrently, resulting in increased acyclovir serum and cerebrospinal fluid [CSF] concentrations, prolonged elimination half-life in the serum and CSF, and, potentially, increased toxicity)
Laboratory value alterations
The following have been selected on the basis of their potential clinical significance (possible effect in parentheses where appropriate;not necessarily inclusive (>> = major clinical significance):
With physiology/laboratory test values
>> Blood urea nitrogen (BUN) and
>> Creatinine, serum:(concentrations may be increased because of renal tubular obstruction caused by intravenous acyclovir; no increase generally occurs with proper dosage and adequate hydration1)
Medical considerations/Contraindications
The medical considerations/contraindications included have been selected on the basis of their potential clinical significance (reasons given in parentheses where appropriate;not necessarily inclusive (>> = major clinical significance).
Risk-benefit should be considered when the following medical problems exist
>> Dehydration or
>> Renal function impairment, pre-existing1:(intravenous acyclovir may increase the potential for nephrotoxicity; it is recommended that acyclovir be administered in a reduced dosage to patients with impaired renal function)
Hypersensitivity to acyclovir or ganciclovir:
Neurological abnormalities or
Prior neurologic reactions to cytotoxic medications1:(intravenous acyclovir may increase the potential for neurologic side effects)
Patient monitoring
The following may be especially important in patient monitoring (other tests may be warranted in some patients, depending on condition; >> = major clinical significance):
Papanicolaou (Pap) test9:(although a clear association has not been shown to date, patients with genital herpes may be at increased risk of developing cervical cancer; Pap test should be done at least once a year to detect early cervical changes)
>> Blood urea nitrogen (BUN) and >> Creatinine, serum1:(concentrations required prior to and during therapy since intravenous acyclovir may be nephrotoxic; if acyclovir is given by rapid intravenous injection or its urine solubility is exceeded, precipitation of acyclovir crystals may occur in renal tubules; renal tubular damage may occur and may progress to acute renal failure)
Side/Adverse Effects
Note: Acute renal insufficiency may occur due to precipitation of acyclovir in the renal tubules. It is most likely to occur if acyclovir is given by rapid intravenous injection, concurrently with known nephrotoxic medications, to patients who are inadequately hydrated, or to patients with renal function impairment without appropriate dosage reduction. However, acute renal failure has also been reported in patients receiving oral acyclovir.50,85,86
Neuropsychiatric toxicity has been associated with high plasma acyclovir concentrations:which may occur when high doses are used, or when patients with renal function impairment are not given an appropriately lowered dose. Neuropsychiatric toxicity may also be more likely to occur in immunocompromised patients and geriatric patients.50
The following side/adverse effects have been selected on the basis of their potential clinical significance (possible signs and symptoms in parentheses where appropriate;not necessarily inclusive:
Those indicating need for medical attention
Incidence more frequent2,49,50
For parenteral acyclovir
Phlebitis or inflammation at the injection site (pain, swelling, or redness)
Incidence less frequent2,49,50
For parenteral acyclovir:more common with rapid intravenous injectionAcute renal failure (abdominal pain; decreased frequency of urination or amount of urine; increased thirst; loss of appetite; nausea; vomiting; unusual tiredness or weakness)
Incidence rare
For parenteral acyclovir only
Encephalopathic changes (coma; confusion; hallucinations; seizures; tremors)
Those indicating need for medical attention only if they continue or are bothersome
Incidence more frequent:especially with high doses
For parenteral acyclovir
Gastrointestinal disturbances (loss of appetite; nausea or vomiting); lightheadedness
Incidence less frequent:with long-term use or high doses
For oral acyclovir
Gastrointestinal disturbances (nausea or vomiting; diarrhea; abdominal pain); headache; lightheadedness
Patient Consultation
As an aid to patient consultation, refer to Advice for the Patient, Acyclovir (Systemic).
In providing consultation, consider emphasizing the following selected information (>> = major clinical significance):
Before using this medication
>> Conditions affecting use, especially:
Hypersensitivity to acyclovir or ganciclovir
Pregnancy:Acyclovir crosses the placenta
Breast-feeding:Acyclovir is distributed into breast milk at concentrations from 0.6 to 4.1 times the corresponding plasma concentration
Use in children:Neonates have an age-related decrease in acyclovir clearance
Other medications, especially nephrotoxic medications
Other medical problems, especially dehydration or pre-existing renal function impairment
Proper use of this medication
Supplying patient information about herpes simplex or varicella-zoster infections
For treatment of recurrent herpes simplex infections, initiating use of the medication as soon as possible after symptoms of recurrence begin to appear
For treatment of chickenpox (varicella), initiating use of oral acyclovir at the earliest sign or symptom; it is most effective when started within 24 hours of the onset of a typical chickenpox rash
Capsules, tablets, and oral suspension may be taken with meals
Taking with full glass of water
Proper administration technique for oral liquids
>> Compliance with full course of therapy; not using more often or for longer than prescribed
>> Proper dosingMissed dose: Taking as soon as possible; not taking if almost time for next dose; not doubling doses
>> Proper storage
Precautions while using this medication
>> Women with herpes genitalis may have an increased risk of developing cervical cancer; annual Pap tests may be required9
Checking with physician if no improvement within a few days2
Keeping affected areas as clean and dry as possible; wearing loose-fitting clothing to avoid irritation of lesions9
>> Use of acyclovir has not been shown to prevent the transmission of herpes simplex virus to sexual partners
>> Herpes genitalis may be sexually transmitted even if partner is asymptomatic89; sexual activity should be avoided if either partner has signs and symptoms of herpes genitalis; use of a condom may help prevent transmission of herpes; however, spermicidal jellies or diaphragms probably will not be adequately protective2,9
Side/adverse effects
Signs of potential side effects, especially phlebitis or inflammation at site of injection, acute renal failure, and encephalopathic changes
General Dosing Information
Therapy should be initiated as soon as possible following the onset of signs and symptoms of herpes simplex or varicella zoster infections.
Because it may take longer for lesions to heal in immunocompromised patients (an average of 2 weeks of therapy for herpes simplex infections), the duration of therapy may need to be prolonged beyond the recommended number of days until the lesions are crusted over or epithelialized.50
For oral dosage forms only
Acyclovir capsules, tablets, and oral suspension may be taken with meals since absorption has not been shown to be significantly affected by food.2
Intermittent short-term treatment of recurrent herpes genitalis infections may be effective for some patients, especially when treatment is patient-initiated during the prodrome or first sign of lesion formation.2
For parenteral dosage forms only
Sterile acyclovir sodium should be administered by intravenous infusion only. It should not be administered topically, intramuscularly, orally, subcutaneously, or ophthalmically.1,4
Intravenous infusions of acyclovir should be administered at a constant rate over at least a 1-hour period to avoid renal tubular obstruction. Rapid injection must be avoided since precipitation of acyclovir crystals in the tubules may occur and may result in renal function impairment in up to 10% of patients receiving intravenous acyclovir.1
Obese patients should be dosed based on ideal body weight.67
Since maximum urinary concentrations of acyclovir are achieved within 2 hours, patients receiving intravenous infusions and high oral doses must be adequately hydrated during this period to prevent precipitation of acyclovir in renal tubules.1
The dose of acyclovir should be adjusted so that a dose is repeated after hemodialysis since each 6-hour hemodialysis period results in approximately a 60% reduction in acyclovir plasma concentrations.1
For treatment of adverse effects
Since there is no specific antidote, treatment of adverse effects should be symptomatic and supportive with possible utilization of the following: Adequate hydration to prevent precipitation of acyclovir in the renal tubules.3,28,41,42
Hemodialysis to aid in the removal of acyclovir from the blood, especially in patients with acute renal failure and anuria.3,28,41,42
Oral Dosage Forms
Note: Bracketed uses in the Dosage Forms section refer to categories of use and/or indications that are not included in U.S. product labeling.
ACYCLOVIR CAPSULES
Usual adult and adolescent dose
Genital herpes infections91:
Initial therapy: Oral, 200 mg every four hours while awake, five times a day, for ten days.
Recurrent infections, intermittent therapy: Oral, 200 mg every four hours while awake, five times a day, for five days.
Recurrent infections, chronic suppressive therapy: Oral, 400 mg twice a day; or 200 mg three to five times a day.50,91
Herpes zoster:
Oral, 800 mg every four hours while awake, five times a day, for seven to ten days.44,45,58,66,78
Varicella:
Oral, 20 mg per kg of body weight, up to 800 mg per dose, four times a day for five days. Treatment should be initiated at the earliest sign or symptom of chickenpox.90
[Herpes simplex, mucocutaneous (treatment)]:
Oral, 200 to 400 mg five times a day for ten days in immunocompromised patients.50,61
[Herpes simplex, mucocutaneous (prophylaxis)]*:
Oral, 400 mg every twelve hours.93
Note: Adults with acute or chronic renal impairment require a reduction in dose.
The recommended dose for initial therapy and intermittent therapy of herpes infections in patients with renal function impairment is:83
Creatinine Clearance Dose
Dosing Interval
(mL/min)/(mL/sec) (mg) (hr)
Genital herpes:
Initial/intermittent therapy
>10/0.17 200
4 (5 times daily)
0-10/0-0.17 200
12
Chronic suppressive
>10/0.17 400
12
0-10/0-0.17 200
12
Herpes zoster (shingles):
>25/0.42 800
4 (5 times daily)
10-25/0.17-0.42 800
8
0-10/0-0.17 800
12
Usual pediatric dose
Children up to 2 years of age:Dosage has not been established90. However, no unusual toxicity or pediatrics-specific problems have been observed in studies done in children using doses of up to 3000 mg per square meter of body surface area per day and 80 mg per kg of body weight per day.55,68,69,70,71,72
Children 2 to 12 years old:Varicella: Oral, 20 mg per kg of body weight, up to 800 mg per dose, four times a day for five days. Treatment should be initiated at the earliest sign or symptom of chickenpox.90
Strength(s) usually available
U.S.:
200 mg (Rx)[Zovirax (lactose)].
Canada:
200 mg (Rx)[Avirax].[Zovirax (lactose) (parabens)].
Packaging and storage:
Store between 15 and 25 ;C (59 and 77 ;F), in a tight container, unless otherwise specified by manufacturer. Protect from light and moisture.2,78
Auxiliary labeling:
Continue medicine for full time of treatment.
ACYCLOVIR ORAL SUSPENSION
Usual adult and adolescent dose
See Acyclovir Capsules.
Usual pediatric dose
See Acyclovir Capsules.
Strength(s) usually available
U.S.:
200 mg per 5 mL (Rx)[Zovirax].
Canada:
200 mg per 5 mL (Rx)[Avirax].[Zovirax].
Packaging and storage:
Store between 15 and 25 ;C (59 and 77 ;F), in a tight container, unless otherwise specified by manufacturer. Protect from light.2,78
Stability:
Suspension retains its potency for 24 months from date of manufacture. Does not require reconstitution or refrigeration.79
Auxiliary labeling:
Continue medicine for full time of treatment.
Shake well.
Take with water.
Beyond-use date.
Note: When dispensing, include a calibrated liquid-measuring device.
ACYCLOVIR TABLETS
Usual adult and adolescent dose
See Acyclovir Capsules.
Usual pediatric dose
See Acyclovir Capsules.
Strength(s) usually available
U.S.:
400 mg (Rx)[Zovirax].800 mg (Rx)[Zovirax].
Canada:
200 mg (Rx)[Avirax].[Zovirax (lactose)].400 mg (Rx)[Avirax].[Zovirax (lactose)].800 mg (Rx)[Avirax].[Zovirax (lactose)].
Packaging and storage:
Store between 15 and 25 ;C (59 and 77 ;F), in a tight container, unless otherwise specified by manufacturer. Protect from light.
Auxiliary labeling:
Continue medicine for full time of treatment.
Parenteral Dosage Forms
Note: Bracketed uses in the Dosage Forms section refer to categories of use and/or indications that are not included in U.S. product labeling.
Note: The dosing and strength of the dosage forms available are expressed in terms of acyclovir base.
ACYCLOVIR SODIUM STERILE
Usual adult and adolescent dose
Genital herpes infections, severe, initial: Intravenous infusion, 5 mg (base) per kg of body weight every eight hours for five days. Administer at a constant rate over at least a one-hour period.1
Herpes simplex (HSV-1 and HSV-2) infections, mucocutaneous, in immunocompromised patients: Intravenous infusion, 5 to 10 mg (base) per kg of body weight every eight hours for seven to ten days. Administer at a constant rate over at least a one-hour period.1,15
Herpes simplex encephalitis*: Intravenous infusion, 10 mg (base) per kg of body weight every eight hours for ten days. Administer at a constant rate over at least a one-hour period.16,17,18,67
Varicella zoster in immunocompromised patients: Intravenous infusion, 10 mg (base) per kg of body weight every eight hours for seven days. Administer at a constant rate over at least a one-hour period.21,50,67
Note: Adults with acute or chronic renal impairment require a reduction in dose and/or dosing interval as follows:1
Creatinine Clearance Dose
Dosing Interval
(mL/min)/(mL/sec) (base) (hr)
>50/0.83 100%
8
25-50/0.42-0.83 100%
12
10-25/0.17-0.42 100%
24
0-10/0-0.17 50%
24
Usual adult prescribing limits
Up to 30 mg (base) per kg of body weight or 1.5 grams per square meter of body surface daily.15
Usual pediatric dose
Herpes genitalis infections, severe, initial: Intravenous infusion, Infants and children up to 12 years of age:250 mg (base) per square meter of body surface every eight hours for five days. Administer at a constant rate over at least a one-hour period.1,60
Children 12 years of age and over:See Usual adult and adolescent dose.
Herpes simplex (HSV-1 and HSV-2) infections, mucocutaneous, in immunocompromised patients:
Infants and children up to 12 years of age:Intravenous infusion, 250 mg (base) per square meter of body surface every eight hours for seven days. Administer at a constant rate over at least a one-hour period.1,60
Children 12 years of age and over:See Usual adult and adolescent dose.
Herpes simplex encephalitis*: Intravenous infusion, 10 mg (base) per kg of body weight, or 500 mg per square meter, every eight hours for ten days. Administer at a constant rate over at least a one-hour period.19,20,67
Varicella zoster in immunocompromised children: Intravenous infusion, 500 mg (base) per square meter every eight hours for seven days. Administer at a constant rate over at least a one-hour period.50,64,67
[Disseminated HSV in neonates]*: Intravenous infusion, 10 mg (base) per kg of body weight every eight hours for ten to fourteen days. Administer at a constant rate over at least a one-hour period.61
Strength(s) usually available
U.S.:
500 mg (base) (Rx)[Zovirax].1 gram (base) (Rx)[Zovirax].
Canada:
500 mg (base) (Rx)[Zovirax].1 gram (base) (Rx)[Avirax].
Packaging and storage:
Prior to reconstitution, store between 15 and 30 ;C (59 and 86 ;F), unless otherwise specified by manufacturer.1
Preparation of dosage form:
To prepare initial dilution for intravenous infusion, add 10 or 20 mL of sterile water for injection or bacteriostatic water for injection to each 500-mg or 1-gram vial, respectively, to provide a concentration of 50 mg per mL.66 Do not use bacteriostatic water for injection containing benzyl alcohol.67 To ensure complete dissolution, shake vial well until solution is clear. The resulting solution should be further diluted with a suitable diluent (standard electrolyte- and dextrose-containing solutions) to at least 100 mL. Final concentrations of 7 mg per mL or less are recommended. Higher concentrations (e.g., 10 mg per mL) may cause phlebitis or inflammation at the injection site upon inadvertent extravasation.1
Stability:
After reconstitution with sterile water for injection, solutions at concentrations of 50 mg per mL retain their potency for 12 hours at controlled room temperature (15 to 25 ;C [59 to 77 ;F]).
After further dilution with standard electrolyte- and dextrose-containing solutions for intravenous infusion, solutions retain their potency for 24 hours at controlled room temperature (15 to 25 ;C [59 to 77 ;F]).
Refrigeration of reconstituted solutions may result in the formation of a precipitate, which will redissolve when warmed to room temperature.1
Incompatibilities:
Sterile acyclovir sodium is incompatible with biological or colloidal solutions (e.g., blood products, protein-containing solutions).
Parabens are incompatible with sterile acyclovir sodium and may cause precipitation.1,39
*Not included in Canadian product labeling.
=============================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No (64) 31/05/2025 DR. JOSÉ LAPENTA R.
===================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.025
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.025
Tlf: 0414-2976087 - 04127766810














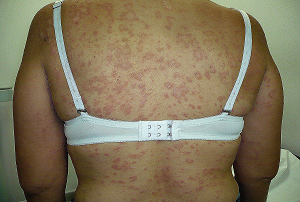




.png)