LOS HONGOS Y SUS FAMILIAS
En el Reino Fungi se puede encontrar una amplia variedad de formas de vida, como mohos, levaduras y hongos. Aproximadamente el 5% de las más de 1,5 millones de especies de hongos que existen en el planeta han sido clasificadas. Se han establecido seis grandes grupos o familias que abarcan la mayoría de las especies conocidas, lo que ha supuesto un reto para los científicos.
La mayoría de los hongos han sido clasificados en 6 (seis) familias o grupos, que abarcan múltiples especies:
1. Ascomicetos (Ascomycota)
2. Basidiomicetos (Basidiomycota)
3. Zigomicetos (Zygomycota):
4. Quitridiomicetos (Chytridiomycota)
5. Glomeromicetos (Glomeromycota)
6.) Deuteromycetes (Fungi Imperfecti):
Como ejemplo en la foto te presento el "famoso hongo del pan" de la familia zigomicetos, el cual es muy común ver en el pan envejecido.
Aqui te dejo 32 referencias Bibliográficas sobre los hongos y sus familias.
Saludos,,,
Dr. José Lapenta.
ENGLISH
A wide variety of life forms can be found in the Fungi Kingdom, such as molds, yeasts, and mushrooms. Approximately 5% of the more than 1.5 million species of fungi that exist on the planet have been classified. Six large groups or families have been established that encompass most of the known species, which has been a challenge for scientists.
Most fungi have been classified into 6 (six) families or groups, which include multiple species:
1. Ascomycetes (Ascomycota)
2. Basidiomycetes (Basidiomycota)
3. Zygomycetes (Zygomycota):
4. Chytridiomycetes (Chytridiomycota)
5. Glomeromycetes (Glomeromycota)
6.) Deuteromycetes (Fungi Imperfecti):
As an example in the photo I present the "famous bread fungus" of the zygomycetes family, which is very common to see in stale bread.
Here I left 32 bibliographical references on fungi and their families.
Greetings...
Dr. José Lapenta R.
======================================================================
DERMAGIC/EXPRESS(42)
======================================================================
EL HONGO Y SUS FAMILIAS / THE FUNGI AND THEIR FAMILIES
======================================================================
1.) THE FUNGI
2.) AGARICACEAE
3.) URICULARIACEAE
4.) BOLETACEAE
5.) CLAVICIPITACEAE
6.) COLEOSPORIACEAE
7.) CONIOPHORACEAE
8.) COPRINACEAE
9.) CORTINARIACEAE
10.) Subdivision DEUTEROMYCOTINA (Fungi Imperfecti)
11.) ENTOMOPHTHORACEAE
12.) GOMPHACEAE
13.) GYMNOASCACEAE
14.) HELVELLACEAE
15.) HYPOCREACEAE
16.) MICROASCACEAE
17.) MORCHELLACEAE
18.) MUCORACEAE
19.) PHAEOSPHAERIACEAE
20.) PIEDRAIACEAE
21.) PYRENOPHORACEAE
22.) RUSSULACEAE
23.) SACCHAROMYCETACEAE
24.) SAKSENAEACEAE
25.) SCHIZOPHYLLACEAE
26.) SCLEROTINIACEAE
27.) TRICHOCOMACEAE
28.) USTILAGINACEAE
29.) ZOPFIACEAE
30.) DERMATOLOGY ASPECTS
31.) VETERINARY ASPECTS
32.) REFERENCES
======================================================================
======================================================================
1.) THE FUNGI
======================================================================
Source: Richard J. Schmidt PhD
Fungi are non-photosynthesising, heterotrophic organisms that derive their energy from a saprophytic or parasitic existence. They are unicellular, amoeboid, or filamentous, never having the leaves, stems, and roots characteristic of higher plants. Reproduction occurs by sexual or asexual spore formation.
Increasingly, it is becoming evident that mycologists regard the fungi as being distinct from plants, and accordingly that they should be classified within their own kingdom, namely the Fungi or Myceteae (Talbot 1971, Cooper 1985, Hawksworth et al. 1983, Holmes 1983). Two divisions of the Fungi are recognised: the Myxomycota (the slime moulds or slime fungi) and the Eumycota (the true fungi). The Myxomycota is further subdivided into four classes: Acrasiomycetes, Hydromyxomycetes, Myxomycetes, and Plasmidiophoromycetes. The Eumycota consists of the so-called lower fungi, or moulds, and the higher fungi amongst which are included the mushrooms and toadstools. The lower fungi, commonly known as the Phycomycetes, are now classified in two subdivisions, namely the Mastigomycotina and the Zygomycotina. The higher fungi, commonly known as the Ascomycetes and the Basidiomycetes, are classified in a further two subdivisions, namely the Ascomycotina and the Basidiomycotina. It should be noted that alternative classifications may be found in the literature.
A fifth subdivision within the Eumycota is named the Deuteromycotina or Fungi Imperfecti; fungi that are known only to reproduce asexually are classified therein. Strictly, taxonomic ranks within the Deuteromycotina should be prefixed with the term "form-", as in "form-genus", "form-class", "form-order", etc. This is because classification is based on outward appearance only since characteristics concerned with sexual reproduction are unknown (Holmes 1983). On occasion, a fungus classified in the Deuteromycotina is persuaded to undergo sexual reproduction, and therefore to show its "perfect" state, whence it becomes evident that it should be reclassified within another subdivision, usually the Ascomycotina or less commonly the Basidiomycotina. When such an event occurs, the fungus has to be provided with a new name. It should be noted that both names remain valid and their usage is determined by the morphological state in which the fungus is being referred to. Contemporary terminology refers to the imperfect states as anamorphs, and to the perfects states as teleomorphs (see Domsch et al. 1980).
One of the purposes of considering the higher plants as species within genera belonging to families is to enable some predictions to be made as to the hazard that any particular plant is likely to present, on the basis that related plants are likely to contain similar phytochemicals. It should be evident, therefore, that classification of the fungi imperfecti into form-families does not produce any useful information concerning genetic relationships between individual form-species or even (but to a lesser extent) form-genera. This may be illustrated by the observation that the conidial (i.e. imperfect) forms of Nectria Fr. (fam. Nectriaceae) are distributed among 14 form-genera and four form-families of the Deuteromycotina (Talbot 1971). For this reason, the fungi imperfecti are considered below as individual form-species within form-genera in the subdivision Deuteromycotina (which is included in its correct alphabetical position as if it were a family), with no attempt being made to classify into form-families. Many form-family names may be encountered in the literature, including Bactridiaceae, Conidiosporiaceae, Cryptococcaceae, Dematiaceae, Excipulaceae, Geotrichaceae, Helminthosporiaceae, Leptostromataceae, Melanconiaceae, Moniliaceae, Mucedinaceae, Sphaeropsidaceae, Sporobolomycetaceae, Stilbellaceae (or Stilbaceae), and Tuberculariaceae. These have not been used in this text.
Fungi have many economic uses and human contact with either the fungi or their secondary metabolites occurs commonly. Several species are often used for food, for example Agaricus bisporus Pilát (the cultivated mushroom), A. campestris L. ex Fr. (the field mushroom), and A. arvensis Schaeff. ex Secr. (the horse mushroom), all of the fam. Agaricaceae; the cep (Boletus edulis Bull. ex Fr., fam. Boletaceae); the Japanese "shiitake" (Lentinula edodes Pegler) and "matsutake" (Tricholoma matsutake, both of the fam. Tricholomataceae); Volvariella esculenta Singer, fam. Pluteaceae, a South-East Asian species; truffles belonging to the genera Tuber Mich., Terfezia Tul. (both of the fam. Terfeziaceae) and Stephensia Tul. & C. Tul. (syn. Elderia McLennan), fam. Humariaceae; and morels (Morchella St Amans spp., fam. Morchellaceae). A number of species are utilised in the manufacture of various foods and beverages. Certain Penicillium Link ex Fr., Rhizopus Ehrenb., Aspergillus Mich. ex Fr. (all of the subdivision Deuteromycotina) and Saccharomyces Meyen ex E. Hansen (fam. Saccharomycetaceae) species are valuable in this respect (see Talbot 1971). The use of the giant puff ball (Langermannia gigantea Rostk., syns Lycoperdon bovista L., Lycoperdon giganteum Batsch.) as a haemostatic is noted by Wren (1968).
Inhalation of fungal spores from various species can produce allergic alveolitis (McCombs 1972, Seaton & Morgan 1984). Towey et al. (1932) described a "coniosporiosis" of timber workers who developed a severe bronchial asthma after inhaling the spores of the deuteromycete Cryptostroma corticale Gregory & Walker (syn. Coniosporium corticale Ellis & Everhart) from infected maple (Acer L. spp., fam. Aceraceae) logs.
A number of medicinally used antibiotics are derived from fungi (Reynolds 1982, Domsch et al. 1980). Penicillins are derived from the deuteromycete Penicillium chrysogenum Thom (syn. P. notatum Westling). Most of the more modern penicillins are produced semi-synthetically from 6-aminopenicillanic acid derived from the Penicillium culture. A related group of antibiotics, the cephalosporins, are derived from cultures of another deuteromycete Acremonium chrysogenum W. Gams (syn. Cephalosporium acremonium Corda), the active nucleus in this group being 7-aminocephalosporanic acid. Fusidium coccineum Fuckel (subdivision Deuteromycotina) is the source of fusidic acid, an antibiotic with a steroid structure. Other less well known antibiotics include adicillin from Emericellopsis salmosynnematum Groskl. & Swift (provisionally classified in the fam. Pseudoeurotiaceae) and fusafungine from Fusarium lateritium Nees ex Link (teleomorph: Gibberella baccata Sacc., fam. Nectriaceae).
The pathogenicity of certain fungi, often manifesting itself as a skin disorder, is perhaps the commonest form of interaction between man and members of the fungus kingdom. This aspect is covered in the monographs below but interested readers are advised to consult an appropriate clinical mycology text for more detailed coverage. Fungal infections acquired from contact with plant material also occur fairly commonly. In particular, sporotrichosis (see Sporothrix schenckii Hektoen & Perkins, subdivision Deuteromycotina) should be suspected in patients with subcutaneous lesions who handle thorny plants, timber, or sphagnum moss (Kaufman 1980). Otherwise, both irritant and allergenic reactions have been reported following contact with various fungi or their extractives. No one group of compounds can be incriminated as being a characteristic cause of a fungus-induced dermatosis. Dermatological effects following deliberate or inadvertent ingestion of certain fungi have also been reported. These include ergotism from Claviceps purpurea Tul. (fam. Clavicipitaceae) and the disulfiram-like reaction following the ingestion of alcohol and ink cap fungi (Coprinus atramentarius Fr., fam. Coprinaceae).
========================================================================
2.) FUNGI - AGARICACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
Agaricus bisporus Pilát
Workers in canneries who prepare mushrooms are subject to keratitis, lachrymation, and other ocular affections accompanied by such constitutional symptoms as vomiting and jaundice. Helvellic acid which becomes vaporised in the air when the mushrooms are washed in cold water is believed to be the cause (Schwartz et al. 1957), but a more likely culprit is p-hydroxymethyl phenylhydrazine (Chilton 1978).
------------------------------------------------------------------------
Agaricus campestris L. ex Fr.
These mushrooms were reported to be the cause of contact dermatitis of the hands and face in a male mushroom grower. Patch tests implicated the mushroom rather than the nicotine-containing insecticide used (Hopkins 1952, 1953).
Inhalation of fungal particles from mushroom compost can produce mushroom worker's lung, a form of allergic alveolitis (Seaton & Morgan 1984).
========================================================================
3.) FUNGI - AURICULARIACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
--------------------------------------------------------------------------------
Auricularia polytricha Sacc.
Black Tree Fungus, Mo-Er
This fungus is used as a texture food in many Szechwanese and Mandarin dishes. It is cultivated on oak (Quercus L., fam. Fagaceae) poles in China (Hawksworth et al. 1983).
Hammerschmidt (1980) described a patient in whom ingestion of a large quantity of the fungus produced a transient inhibition of platelet aggregation resulting in purpura and epistaxis.
========================================================================
4.) FUNGI - BOLETACEAE
========================================================================
This family is classified within the subdivision Basidiomycotina. A distinguishing feature of members of this family is the presence of pores in place of gills on the fruiting bodies (mushrooms). Many of the boletes are edible (Grimes 1978).
---------------------------------------------------------------
Boletus luteus L. ex Fr.
Two cases of cutaneous sensitisation to Boletus luteus were reported; one patient also showed a weakly positive reaction to B. edulis Bull. ex Fr. (Hellerström 1941). See also Lactarius deliciosus Fr., fam. Russulaceae and Ramaria flava Quélet, fam. Gomphaceae.
========================================================================
5.) FUNGI - CLAVICIPITACEAE
========================================================================
This family of about 224 species in 23 genera is classified in the subdivision Ascomycotina.
--------------------------------------------------------------------------------
Claviceps Tul.
Ergot
Members of this genus are pathogens of various grasses and cereals, forming sclerotia known commonly as ergots. Inadvertent ingestion of the sclerotia of certain Claviceps in flour prepared from infected cereal crops can produce gangrene of the extremities, often referred to as St Anthony's fire or ergotism (Sollman 1957). C. purpurea Tul., perhaps the most common species, is the original source of ergotamine, ergometrine, and other ergot alkaloids used in medicine (Talbot 1971, Robbers et al 1996).
========================================================================
6.) FUNGI - COLEOSPORIACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
--------------------------------------------------------------------------------
Coleosporium Lév.
The genus is one of the 100 or so genera of fungi known as rusts (see also Pucciniaceae below) and classified in the order Uredinales (Talbot 1971). The genus has also been classified in the family Melampsoraceae (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Coleosporium sonchi Lév. & Tul.
(syns Coleosporium sonchi-arvensis Lév., Uredo sonchi-arvensis Pers.)
Pammel (1911) lists this species as an irritant.
========================================================================
7.) FUNGI - CONIOPHORACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
Serpula lacrimans Schröter
(syn. Merulius lacrimans Fr.)
Dry Rot
This well known wood-rotting fungus has also been classified in the family Meruliaceae.
White (1934) observed two adult males who suddenly developed an erythematous eruption on the face and exposed parts of the hands and arms following sweeping out a cellar containing a dry decaying wood. The eruptions subsided in 36 hours. He stated that the "dry rot" fungus was the probable irritant. It should be noted, however, that other species of wood-decaying fungus may be found in such a situation. Frankland & Hay (1951) also refer to allergic complaints from the dry rot fungus.
========================================================================
8.) FUNGI - COPRINACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
--------------------------------------------------------------------------------
Coprinus atramentarius Fr.
Ink Cap
Although normally an edible fungus, poisoning occurs if alcohol is consumed upto 48 hours after ingestion. The symptoms are reddening of the face and difficulty in breathing, and resemble disulfiram poisoning. The compound responsible is coprine (Chilton 1978). The onset of symptoms varies from 20 minutes to 2 hours after consumption of alcohol (North 1967).
========================================================================
9.) FUNGI - CORTINARIACEAE
========================================================================
The family is classified in the subdivision Basidiomycotina.
--------------------------------------------------------------------------------
Hebeloma crustuliniforme Quéelet
Poison Pie, Fairy-Cake Mushroom
This fungus smells of radish (North 1967). Whether or not isothiocyanates are responsible is unknown. See also Cruciferae.
========================================================================
10.) FUNGI - Subdivision DEUTEROMYCOTINA
========================================================================
(Fungi Imperfecti)
------------------------------------------------------------------------
Acremonium Link ex Fr.
Many of the organisms hitherto placed in the form-genus Cephalosporium Corda are now considered to be species of Acremonium Link ex Fr. The name Cephalosporium acremonium Corda referred to commonly in the medical literature is actually of uncertain application. The vast majority of reports referring to the production of the antibiotic cephalosporin C by C. acremonium refer to Acremonium chrysogenum W. Gams; the C. acremonium that may cause mycetomas is now called Acremonium kiliense Grütz (Domsch et al. 1980).
The following organisms have been identified as causes of mycetomas (Padhye & Ajello 1980, Domsch et al. 1980):
A. falciforme Gams
(syn. Cephalosporium falciforme Carrión)
A. kiliense Grütz
A. recifei Gams
(syn. Cephalosporium recifei Leão & Lôbo)
A. kiliense and A. potroni auct. may cause eye infections (Rebell & Forster 1974, 1980).
The teleomorphic genera Nectria Fr. (fam. Nectriaceae), Emericellopsis Van Beyma (provisionally classified in the fam. Pseudoeurotiaceae), and Mycoarachis Malloch & Cain (provisionally classified in the fam. Cephalothecaceae) have been identified with certain Acremonium species (Domsch et al. 1980).
--------------------------------------------------------------------------------
Acremonium chrysogenum W. Gams
This organism provides 7-aminocephalosporanic acid from which semi-synthetic cephalosporin-type antibiotics are produced. A disulfiram reaction (see also Coprinus atramentarius Fr., fam. Coprinaceae) has been reported to occur following the ingestion of alcohol and certain cephalosporin-type antibiotics. The reaction appears to occur only with those compounds possessing the methyltetrazolethiol group at C-3, for example latamoxef, cefoperazone, and cephamandole (Stockley 1985).
Rudzki & Rebendel (1984) observed 3 positive patch test reactions to cefradine among 20 pharmaceutical workers and nurses screened for sensitivity to this antibiotic.
Tuft (1975) described the occurrence of contact urticaria in a chemist working with cephalosporins. Epicutaneous challenge tests with cephalothin sodium and other cephalosporins (unnamed) produced whealing.
--------------------------------------------------------------------------------
Alternaria Nees ex Fr.
Members of this form-genus of some 44 species are common plant parasites, causing leaf spot and stem rot diseases (Martin 1969). They are occasionally implicated as agents of phaeohyphomycosis (see also Phialophora Medlar) and have been associated with infections involving bone, cutaneous tissue, ears, eyes, and urinary tract. Only A. alternata Keissler appears to have been positively identified in this respect (McGinnis 1980).
--------------------------------------------------------------------------------
Alternaria alternata Keissler
(syns Alternaria tenuis Nees, Torula alternata Fr.)
Campbell & White (1989) noted that this organism may cause localised tissue destruction, usually in the nasal region and presumably following germination of inhaled conidia, in patients with AIDS.
A crude physiological saline extract of A. tenuis produced positive patch test reactions in 1 of 6 patients with seasonal eczematous dermatitis; a crude ether extract failed to elicit a response in that patient (Fujisawa et al. 1966).
--------------------------------------------------------------------------------
Arthrinium sphaerospermum Fuckel
(syn. Papularia sphaerosperma von Höhnel)
The "maladie de la cannes de Provence" occurs on the shoulders of workmen who carry bundles of reeds (Arundo donax L., fam. Gramineae) affected by this fungus (Mandoul et al. 1954).
--------------------------------------------------------------------------------
Aspergillus Mich. ex Fr.
Aspergillus species occur commonly in soil, particularly in association with decaying plant material (Domsch et al. 1980). Perfect states of these organisms have been classified in a number of ascomycete genera including Eurotium Link ex Fr., Emericella Berk. & Br., and Neosartorya Malloch & Cain, all in the family Trichocomaceae (Austwick & Longbottom 1980, Domsch et al. 1980).
At least 8 of the 160 or so species of Aspergillus that have been described are known to be pathogenic to humans and to animals. Their pathogenicity is wide ranging: infection may occur within or on the surfaces of affected organs; sensitisation may occur through exposure to spores, hyphae, or metabolites by inhalation, ingestion, or by contact; and toxicosis may occur following ingestion of metabolites such as the aflatoxins or the ochratoxins (Austwick & Longbottom 1980, Roberts et al. 1984).
Autoerythrocyte sensitisation, which apparently resulted from mould antigen absorption onto the red cell, was found responsible for recurrent urticaria (Shelley & Florence 1961). The patient was exquisitely sensitive to trichophytin with a cross-sensitivity to mould-derived antibiotics and the common saprophytes Aspergillus glaucus Link, Aspergillus flavus Link, and an unidentified Alternaria Nees ex Fr. species.
--------------------------------------------------------------------------------
Aspergillus flavus Link ex Gray
This species is a common cause of mycotic ocular infection (Rebell & Forster 1974).
--------------------------------------------------------------------------------
Aspergillus fumigatus Fresen.
(syn. Aspergillus nigrescens Robin)
This species is the main cause of aspergillus infection and allergy. It is often found as a cause of infection of the turbinate and ethmoid regions of the nose (Austwick & Longbottom 1980). A. fumigatus is a major cause of exogenous keratomycosis (Rebell & Forster 1974).
--------------------------------------------------------------------------------
Aspergillus terreus Thom
Aspergillus species, and especially A. terreus, are the commonest cause of fungal infections of the ear. The organisms have also been found in subcutaneous abscesses and may invade the nails (Austwick & Longbottom 1980).
--------------------------------------------------------------------------------
Aspergillus versicolor Tiraboschi
A crude physiological saline extract of this organism produced positive patch test reactions in a patient with seasonal eczematous dermatitis; a crude ether extract failed to elicit a response (Fujisawa et al. 1966).
--------------------------------------------------------------------------------
Candida Berkhout
Yeasts belonging to this form-genus are non-pigmented and have the capacity to form pseudomycelium in culture. Some can also form true mycelium. C. albicans Berkhout and C. glabrata S.A. Meyer & Yarrow are commonly found as commensal organisms in the gut of man, animals, and birds (Roberts et al. 1984).
Some Candida species are capable of producing opportunistic infections known variously as candidiasis, candidosis, thrush, and moniliasis. The disease may be mild, severe, or chronic, affecting the skin, nails, and mucous membranes including the eyes (Rebell & Forster 1974, Silva-Hutner & Cooper 1980), and may become systemic in immunosuppressed patients (Roberts et al. 1984).
The following species may produce disease (Roberts et al. 1984):
C. albicans Berkhout
(syns Monilia albicans Zopf, Oidium albicans Robin)
C. glabrata S.A. Meyer & Yarrow
(syns Torulopsis glabrata Lodder & De Vries, Cryptococcus glabratus Anderson)
C. krusei Berkhout
(syn. Saccharomyces krusei Castellani)
C. parapsilosis Langeron & Talice
(syn. Monilia parapsilosis Ashf.)
C. pseudotropicalis Basgal
(syns Endomyces pseudotropicalis Castellani, Mycocandida pseudotropicalis Cif. & Red.)
C. stellatoidea Langeron & Guerra
(syn. Monilia stellatoidea Jones & Martin)
C. tropicalis Berkhout
(syn. Oidium tropicalis Castellani)
The vast majority of infections are caused by C. albicans.
Candida allergy produced skin and systemic disease (Joseph et al. 1965). Oidomycin, an extract of Candida, produced positive delayed intracutaneous test reactions in sensitised persons.
--------------------------------------------------------------------------------
Cercospora apii Fr.
Emmons et al. (1957) described verrucous lesions of the face of a 12 year old Indonesian boy, from which this organism was isolated.
--------------------------------------------------------------------------------
Cercosporella Sacc.
Members of this form-genus cause leaf-spot diseases of some plants (Martin 1969). An eruption of the wrists of women sorting and packing dried fruits from the Orient was attributed to a mould from this form-genus. The wrists rubbed against the sacking on the tables containing the fruit (Russ 1923).
--------------------------------------------------------------------------------
Cladosporium Link ex Fr.
Cladosporium species, of which some 500 have been described, are amongst the most common air-borne fungi, and thus have a world wide distribution. They are particularly common on dying or dead plant material. Some have been identified with the teleomorphic genus Mycosphaerella Johanson, a loculoascomycete of the family Dothideaceae (Domsch et al. 1980).
Cladosporium species are occasionally reported from cutaneous, eye, and nail infections (McGinnis 1980).
--------------------------------------------------------------------------------
Cladosporium bantianum Borelli
(syn. Torula bantiana Sacc.)
This organism may cause cladosporiosis (Rogers 1980). It may cause subcutaneous phaeohyphomycosis (McGinnis 1983) and is the most frequently reported aetiological agent of cerebral phaeohyphomycosis (McGinnis 1980).
--------------------------------------------------------------------------------
Cladosporium carrionii Trejos
(syn. Cladophialophora ajelloi Borelli)
This form-species is a causative organism of chromoblastomycosis (Vollum 1977, McGinnis 1980, Roberts et al. 1984). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Coccidioides immitis Stiles in Rixford & Gilchrist
The taxonomic position of this organism has not been unequivocally determined but a relationship with the Gymnoascaceae has been suggested (Domsch et al. 1980).
The vegetative phase of this fungus is highly infective and can cause coccidioidomycosis, a systemic mycosis which may involve the skin and bone as well as the lungs (Domsch et al. 1980). It is endemic to arid regions of North and South America (Ainsworth & Austwick 1973, Roberts et al. 1984). Most patients develop a hypersensitivity state that is readily demonstrated by intradermal coccidioidin skin tests (Larsh & Goodman 1980).
--------------------------------------------------------------------------------
Corynespora cassiicola Wei
(syn. Helminthosporium cassiicola Berk. & Curt.)
This organism has been reported to cause mycetomas (Padye & Ajello 1980).
--------------------------------------------------------------------------------
Curvularia Boedijn
Curvularia species have been implicated in a number of opportunistic infections, and may particularly cause mycotic ulcers (Rebell & Forster 1974, McGinnis 1980, 1983). The following may be regarded as pathogenic:
C. pallescens Boedijn
C. senegalensis Subram.
C. verruculosa Tandon & Bilgrami ex M.B. Ellis
Other Curvularia species are considered under Cochliobolus Drechsler, fam. Pyrenophoraceae, in which genus the teleomorphs of Curvularia geniculata Boedijn and Curvularia lunata Boedijn are classified.
--------------------------------------------------------------------------------
Cylindrocarpon tonkinense Bugnicourt
Mycotic keratitis caused by this species has been reported (Laverde et al. 1973, Rebell & Forster 1974).
--------------------------------------------------------------------------------
Drechslera Ito
Perfect states of Drechslera species have been classified in the genus Pyrenophora Fr., fam. Pleosporaceae.
Drechslera species have been implicated in a number of opportunistic infections (McGinnis 1980, Rogers 1980) and may cause phaeohyphomycosis (Roberts et al. 1984). See also Phialophora Medlar. The following species are recognised pathogens (McGinnis 1980, 1983):
D. hawaiiensis M.B. Ellis
(syns Drechslera hawaiiensis Subram. & Jain, Helminthosporium hawaiiensis Bugnicourt)
D. rostrata Richardson & Fraser
(syn. Helminthosporium rostratum Drechsler)
--------------------------------------------------------------------------------
Epidermophyton cruris Castellani & Chalm.
This organism may cause tinea cruris and tinea pedis in man (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Epidermophyton floccosum Langeron & Milochevitch
(syn. Epidermophyton inguinale Sabouraud)
This organism is a common causes of ringworm in human skin (Ajello & Padhye 1980, Roberts et al. 1984). See also Microsporum Gruby and Trichophyton Malmsten.
--------------------------------------------------------------------------------
Exophiala jeanselmei McGinnis & Padhye
(syns Phialophora jeanselmei Emmons, Phialophora gougerotii Borelli, Sporotrichum gougerotii Matr., Torula jeanselmei Langeron)
This organism is an occasional cause of mycetomas and chromomycosis in the U.S.A. (McGinnis 1978, 1980, Domsch et al. 1980, Padhye & Ajello 1980, Roberts et al. 1984). See also Phialophora Medlar. According to the classification of Mackinnon et al. (1949), this organism produces the so-called Type III maduromycosis (see Madurella grisea Mackinnon, Ferrada, & Montemayer below).
--------------------------------------------------------------------------------
Exophiala moniliae De Hoog
Exophiala spinifera McGinnis
(syn. Phialophora spinifera Nielsen & Conant)
These organisms have been reported as agents of phaeohyphomycosis causing subcutaneous cysts (McGinnis 1978, 1980). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Exophiala werneckii v. Arx
(syns Cladosporium werneckii Horta, Dematium werneckii Dodge, Pullularia werneckii de Vries, Aureobasidium mansonii Cooke)
This organism is the causative agent of tinea nigra, a superficial phaeohyphomycosis characterised by dark macular patches on the palms or palmar aspects of the wrists and fingers (Ajello & Padhye 1980, McGinnis 1980). The use of the term tinea to describe the disease is misleading since it is not a form of ringworm (Roberts et al. 1984, McGinnis et al. 1985).
There is a great deal of confusion in the literature concerning the taxonomy of Exophiala werneckii and its relationship with Microsporum mansonii Castellani. McGinnis (1979) attempted to clarify the situation and concluded that the confusion arose when a case of pityriasis versicolor was misdiagnosed as tinea nigra. The organism responsible was named Microsporum mansonii by Castellani and later renamed Aureobasidium mansonii by Cooke. However, Cooke was actually naming the causative organism of tinea nigra, so his name is now considered to be a synonym of Exophiala werneckii. Since Castellani's name actually referred to the causative organism of pityriasis versicolor, it is now considered to be a synonym of Malassezia furfur Baillon.
--------------------------------------------------------------------------------
Fonsecaea compacta Carrión
(syn. Hormodendrum compactum Carrión)
Fonsecaea pedrosoi Negroni emend. Carrión
(syn. Hormodendrum pedrosoi Brumpt)
Both species are agents of chromoblastomycosis (Vollum 1978, McGinnis 1980, 1983, Roberts et al. 1984). See also Phialophora Medlar.
The genera Fonsecaea Negroni and Cladosporium Link ex Fr. are difficult to differentiate (Rogers 1980).
--------------------------------------------------------------------------------
Fusarium Link ex Fr.
Over 142 species, varieties, and forms are recognised in this form-genus. Teleomorphic genera include Nectria Fr., Gibberella Sacc., and Calonectria de Not. of the fam. Hypocreaceae, and Plectosphaerella Kleb. of the fam. Trichosphaeriaceae (Domsch et al. 1980).
F. dimerum Penzig (syn. F. episphaeria Snyder & Hansen), F. nivale Ces., Rabenh., and F. oxysporum Schlecht. emend Snyder & Hansen) are occasionally responsible for producing mycotic ulcers of the cornea (Rebell & Forster 1974).
--------------------------------------------------------------------------------
Fusidium coccineum Fuckel
This organism is the source of fusidic acid, an antibiotic (Reynolds 1982). A few isolated cases of contact sentitivity to topically applied sodium fusidate have been reported in the literature (Cronin 1980, Romaguera & Grimalt 1985). See also Mucor ramannianus Moller, fam. Mucoraceae.
--------------------------------------------------------------------------------
Hendersonula toruloidea Nattrass
This organism, a black or grey saprophytic mould that sometimes causes plant disease in tropical and subtropical regions, has been recognised as a cause of skin and nail infections almost identical with those produced by Trichophyton rubrum Sabouraud (Campbell et al. 1973, Roberts et al. 1984).
--------------------------------------------------------------------------------
Lasiodiplodia theobromae Griffon & Maubl.
(syn. Botryodiplodia theobromae Pat.)
This organism may cause mycotic keratitis and onchomycosis (Laverde et al. 1973, McGinnis 1983).
--------------------------------------------------------------------------------
Madurella grisea Mackinnon, Ferrada, & Montemayer
Madurella mycetomatis Brumpt
These organisms can cause "black grain" mycetomas (or maduromycosis) in Latin America and the U.S.A. (Padhye & Ajello 1980, Roberts et al. 1984).
According to the classification of Mackinnon et al. (1949), Type I maduromycosis is produced by M. americana Vuillemin, M. ikedai Gammel, and M. mycetomatis Brumpt whilst Type II maduromycosis is produced by M. grisea.
--------------------------------------------------------------------------------
Malassezia furfur Baillon
(syns Monilia furfur Vuillemin, Pityrosporum furfur Emmons, Binford, & Utz, Pityrosporum orbiculare Gordon, Microsporon furfur Robin, Microsporum mansonii Castellani, Cladosporium mansonii Castellani, Malasezzia ovalis Acton & Panja, Pityrosporum ovale Castellani & Chalmers, Saccharomyces ovalis Bizzozero)
Tinea versicolor or pityriasis versicolor, a mild superficial dermatomycosis that usually manifests itself as slightly raised scaly patches on the neck and torso, is thought to be caused by this organism (Ajello & Padhye 1980, Silva-Hutner & Cooper 1980). The organism, a lipophilic yeast which is a member of the normal flora of the skin, becomes pathogenic when it transforms into a more mycelial form but the factors causing this are not clearly understood (Roberts et al. 1984). The complex polymorphic nature of the organism has only recently been recognised (Salkin & Gordon 1977).
The organism has been associated with blepharitis, dacryocystitis, dandruff, and seborrhoea (Benham 1947, Silva-Hutner & Cooper 1980).
--------------------------------------------------------------------------------
Microsporum Gruby
This genus of about 15 species is responsible for ringworm in a variety of animals, but generally only infrequently or rarely in humans. Perfect states of these organisms are classified in the genus Nannizzia Stockdale (fam. Gymnoascaceae) (Ajello & Padhye 1980) under which heading are considered those with known teleomorphs. See also Epidermophyton Sabouraud and Trichophyton Malmsten.
The following Microsporum species have been reported to have caused ringworm infections in man (Ainsworth & Austwick 1973, Domsch et al. 1980, Roberts et al. 1984):
M. canis Bodin
(syns Microsporum felineum Mewborn, Microsporum lanosum Sabouraud, Microsporum equinum Sabouraud)
M. distortum Di Menna & Marples
M. ferrugineum Ota
--------------------------------------------------------------------------------
Mycocentrospora acerina Deighton
(syn. Centrospora acerina Newhall)
This organism has been reported as a cause of systemic phaeohyphomycosis (Deighton & Mulder 1977, McGinnis 1980, 1983, Roberts et al. 1984). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Myrothecium roridum Tode ex Steudel
Brian et al. (1948) observed positive patch test reactions to the culture filtrate of several strains of this fungus in 2 subjects.
--------------------------------------------------------------------------------
Myrothecium verrucaria Ditm. ex Steudel
(syns Metarrhizium glutinosum S. Pope, Peziza verrucaria Alb. & Schw.)
The fungus has been isolated in the U.S.A. from deteriorated baled cotton and from Maryland soil. It causes rapid decomposition of cellulose. Reports of dermatitis of the hand in workers who handled cotton from dry bales on which a powdery mould was growing (White 1934) could perhaps be ascribed to this organism.
White & Downing (1947) showed that Metarrhizium glutinosum and Myrothecium verrucaria were one and the same organism.
Brian et al. (1947) experienced severe facial inflammation whilst processing large volumes of culture fluid during the isolation of glutinosin (an antifungal agent). The inflammatory activity was attributed to a volatile compound in the culture fluid. Self applied patch tests with the culture fluid produced positive reactions. Bowden & Schantz (1955) isolated three compounds possessing high dermatitic activity from a culture of this organism. Their structures were not elucidated.
--------------------------------------------------------------------------------
Paecilomyces lilacinus Samson
(syn. Penicillium lilacinum Thom)
This organism is a significant cause of keratomycosis and endophthalmitis (Rebell & Forster 1980).
--------------------------------------------------------------------------------
Paracoccidioides brasiliensis De Almeida
This organism can cause paracoccidioidomycosis, a systemic mycosis which may involve the skin and mucous membranes. An hypersensitivity state has been demonstrated in asymptomatic individuals (Larsh & Goodman 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Penicillium Link
Eczematous dermatitis has been attributed to contact with or inhalation of spores of Penicillium species (Bocobo et al. 1954).
--------------------------------------------------------------------------------
Penicillium chrysogenum Thom
(syn. Penicillium notatum Westling)
Cultures of this organism are used to produce natural and semi-synthetic penicillin-type antibiotics (Domsch et al. 1980). Penicillins are well known as potent contact sensitisers, this having been demonstrated in humans and in guinea pigs using a maximisation test. See Cronin (1980) for a more complete review, and Rudzki & Rebendel (1984).
--------------------------------------------------------------------------------
Penicillium citrinum Thom
A crude physiological saline extract of this species elicited positive patch test reactions in 2 of 3 patients with seasonal eczematous dermatitis. A crude ether extract failed to elicit a response (Fujisawa et al. 1966).
--------------------------------------------------------------------------------
Penicillium griseofulvum Dierckx
Penicillium nigricans Bain. ex Thom
These organisms are the source of griseofulvin which is used in the oral treatment of a number of dermatomycoses (Domsch et al. 1980).
Griseofulvin may be an occasional photosensitiser, but clinically this rarely seems to be a problem (Cronin 1980).
--------------------------------------------------------------------------------
Phialophora Medlar
Teleomorphic genera associated with Phialophora species include Pyrenopeziza Fuckel (fam. Dermateaceae), Mollisia P. Karsten (fam. Dermateaceae), Ascocoryne Groves & Wilson (fam. Helotiaceae), Coniochaeta Cooke (fam. Xylariaceae), and Gaeumannomyces v. Arx & Olivier (fam. Gnomoniaceae) (Domsch et al. 1980).
Several species of Phialophora are well recognised as aetiological agents of chromoblastomycosis and phaeohyphomycosis. Chromoblastomycosis (also known as chromomycosis) is a chronic infection caused by dark-coloured yeast-like fungi which involves the dermis and epidermis, and is characterised by a warty, often foul-smelling proliferation of the skin. Phaeohyphomycosis (also known as cystic chromomycosis) is an infection of the skin in which brown-pigmented fungi are present in a hyphal or pseudohyphal form. In subcutaneous tissues, cystic lesions are the most frequently recognised form of infection (Roberts et al. 1984). McGinnis et al. (1985) discuss the nomenclature of chromoblastomycosis and phaeohyphomycosis and give several obsolete synonyms for each disease.
Tschen et al. (1984) described two cases of chromomycosis in which pigmented elements of unidentified fungal material were found within or on embedded wood splinters associated with foreign body reactions. Similar cases were described by Mehregan & Rudner (1980) and by Vollum (1977).
Pathogenic species include (Ajello et al. 1974, Vollum 1977, McGinnis 1978, 1980, 1983, Domsch et al. 1980, Roberts et al. 1984):
P. bubakii Schol-Schwarz
P. gougerotii Borelli
(syn. Sporotrichum gougerotii Matruchot)
P. hoffmannii Schol-Schwarz
(syn. Phialophora mutabilis Scol-Schwarz)
P. parasitica Ajello, Georg, & Wang
P. repens Conant
(syn. Cadophora repens Davidson)
P. richardsiae Conant
(syn. Cadophora richardsiae Nannf.)
P. verrucosa Medlar
(syns Phialophora americana Hughes, Cadophora americana Nannf.)
Phialophora gougerotii has been reported to have caused eye infections (Laverde et al. 1973, McGinnis 1980).
--------------------------------------------------------------------------------
Phoma glomerata Wollenw. & Hochapfel
Several cases of pathogenicity ascribable to this organism have been recorded, including granuloma of the foot, mycoses of the hand, otomycosis, etc. (Domsch et al. 1980).
--------------------------------------------------------------------------------
Phoma herbarum Westend.
(syn. Phoma hibernica Grimes, O'Connor, & Cummins)
P. hibernica has been documented as a cause of phaeohyphomycosis involving subcutaneous tissue (McGinnis 1980, 1983). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Pyrenochaeta romeroi Borelli
This organism is known as a cause of "black grain" mycetomas (Padhye & Ajello 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Rhinocladiella Nannf.
This genus of 12-15 species includes some "cellar-fungi" such as R. cellaris M.B. Ellis and R. ellisii D. Hawksw.
--------------------------------------------------------------------------------
Rhinocladiella aquaspersa Schell, McGinnis, & Borelli
(syn. Acrotheca aquaspersa Borelli)
This organism may rarely cause chromoblastomycosis (McGinnis 1983). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Scopulariopsis Bain.
Organisms belonging to this form-genus have been associated with the teleomorphic genus Microascus Zukal, fam. Microascaceae (Domsch et al. 1980).
Binders and vendors of hay, straw, and rushes, those engaged in putting matting around bottles and in seating cane-bottomed chairs may be affected by fungi. White (1934) speculated that Scopulariopsis koningii Vuill. might be responsible; the fungus occurs in litter, straw, and manure.
--------------------------------------------------------------------------------
Scopulariopsis brevicaulis Bain.
(syn. Penicillium brevicaule Sacc.)
This organism may cause deep-seated gummose ulcers and onchomycosis (Domsch et al. 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Scopulariopsis fusca Zach
This has been named as the causative organism in cases of onchomycosis and dermatomycosis (Domsch et al. 1980).
--------------------------------------------------------------------------------
Scytalidium Pesante
This genus comprises some 7 species of cosmopolitan distribution.
McGinnis et al. (1985) refer to a dermatomycosis caused by the Scytalidium synanamorph of Hendersonula toruloides Nattrass.
--------------------------------------------------------------------------------
Scytalidium hyalinum C. Campbell & Mulder
This organism, a white mould, has been recognised as a cause of skin and nail infections almost identical with those produced by Trichophyton rubrum Sabouraud (Roberts et al. 1984).
--------------------------------------------------------------------------------
Scytalidium lignicola Pesante
This organism may cause cutaneous phaeohyphomycosis (McGinnis 1983).
--------------------------------------------------------------------------------
Spondylocladium Mart. ex Corda
Cross-sensitivity was observed between castor bean dust (Ricinus communis L., fam. Euphorbiaceae) and an unidentified species of Spondylocladium (Panzani 1962).
--------------------------------------------------------------------------------
Sporothrix schenckii Hektoen & Perkins
(syn. Sporotrichum schenckii de Buerm. & Gougerot)
Although the teleomorph of this organism has been identified as Ophiostoma stenoceras Nannf. (syns Ceratocystis stenoceras C. Moreau, Ceratostomella stenoceras Robak), the continuing discussion (Domsch et al. 1980) about this relationship suggests that it may be premature to consider this to be unequivocal. The organism occurs widely in soil and on vegetable matter.
This fungus is the aetiological agent of a chronic subcutaneous lymphatic, or rarely respiratory, mycosis in man and other mammals which has been named sporotrichosis (Domsch et al. 1980, Roberts et al. 1984). An hypersensitivity state to sporotrichin, an experimental antigen derived from the organism, has been demonstrated in some asymptomatic individuals (Larsh & Goodman 1980, Roberts et al. 1984).
According to Schwartz et al. (1957), the reeds of Provence (Arundo; fam. Gramineae), used to make sieves, trellises, and ceilings, when piled in heaps carry a dry white mould (Sporotrichum [sic]) which produces dermatitis in workers who strip the stalks.
Sporotrichosis may result from puncture wounds caused by barberry thorns (see Berberis vulgaris L., fam. Berberidaceae). The disease should be suspected in all persons with subcutaneous lesions who handle thorny plants, timber, or sphagnum moss (Kaufman 1980).
--------------------------------------------------------------------------------
Sporothrix schenkii Hektoen & Perkins var. luriei Ajello & Kaplan
This organism is the causal agent of a cranial osteolysis in South Africa (Domsch et al. 1980).
--------------------------------------------------------------------------------
Stachybotrys chartarum Hughes
(syn. Stachybotrys alternans Bonord.)
This mould may be found on hay. Ether extracts of cultures of the mould have dermatitic properties (Bowden & Schantz 1955).
Mycotoxins found in the mycelium can produce stachybotryotoxicosis in both horses (see VETERINARY ASPECTS) and man (Domsch et al. 1980).
--------------------------------------------------------------------------------
Stenella araguata Syd.
(syns Cladosporium castellani Borelli & Marcano, Cladosporium araguatum v. Arx)
This organism may cause tinea nigra, a superficial phaeohyphomycosis (McGinnis et al. 1985).
--------------------------------------------------------------------------------
Taeniolella stilbospora Hughes
(syn. Torula stilbospora Corda)
This organism may cause a dermatomycosis (McGinnis 1983).
--------------------------------------------------------------------------------
Trichophyton Malmsten
This is a genus of about 20 species, some of which are commonly found as causative agents of ringworm in humans (Ajello & Padhye 1980). Teleomorphs are as yet unknown for many of the pathogenic species, but those that are known belong to the genus Arthroderma Berk. (fam. Gymnoascaceae) under which heading they are considered.
Trichophytin, a commercially produced extract from T. mentagrophytes Blanchard (see Arthroderma benhamiae Ajello & Cheng, fam. Gymnoascaceae) and related dermatophytes, produced positive delayed intracutaneous test reactions in sensitised persons (Ramirez 1930).
Roberts et al. (1984) list the following species (in addition to those described in the monographs below) as being locally important causes of ringworm in man:
T. gourvilii Catanei
T. megninii Blanchard
(syn. Megatrichophyton megnini Neveu-Lemaire)
T. soudanense Joyeux
(syn. Langeronia soudanensis Vanbreuseghem)
T. yaoundei Cochet & Doby-Dubois (nomen nudum)
--------------------------------------------------------------------------------
Trichophyton concentricum Blanchard
Tinea imbricata is caused by this organism. This distinctive dermatophyte disease is restricted to Malaysia, the Pacific, the Far East, and parts of Central and South America (Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichophyton equinum Gedoelst
This organism is a common cause of ringworm in horses, but may also invade human skin (Ainsworth & Austwick 1973, Ajello & Padhye 1980).
--------------------------------------------------------------------------------
Trichophyton erinacei Padhye & Carmichael
(syn. Trichophyton mentagrophytes Blanchard var. erinacei J.M.B. Smith & Marples)
This organism has been reported as a cause of ringworm in hedgehogs, rat, mouse, dog, and man (Ainsworth & Austwick 1973, Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichophyton gallinae Silva & Bentham
(syn. Microsporum gallinae Grigorakis)
Although usually described as a species of Microsporon, Ajello (1968) considers that the organism belongs to the genus Trichophyton. The species is recorded as being pathogenic to man (Ainsworth & Austwick 1974, Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichophyton rubrum Castellani
(syns Trichophyton purpureum Bang, Epidermophyton purpureum C.W. Dodge)
This organism is a common cause of ringworm of the skin and nails (Ajello & Padhye 1980, Roberts et al. 1984). It may also produce a hyperkeratotic form of athlete's foot (Sande & Mandell 1985).
--------------------------------------------------------------------------------
Trichophyton schoenleinii Langeron & Milochevitch
(syn. Achorion schoenleinii Remak.)
Favus, a form of ringworm characterised by heavy cup-shaped crusts (scutulae) and hair invaded throughout its length by hyphae which do not fragment into arthrospores, is produced by this organism (Ainsworth & Austwick 1973, Ajello & Padhye 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichophyton tonsurans Malmsten
This organism is a common cause of epidemic tinea capitis in both children and adults (Ajello & Padhye 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichophyton verrucosum Bodin
(syns Trichophyton album Sabouraud, Trichophyton discoides Sabouraud, Trichophyton ochraceum Sabouraud)
This organism is a common cause of ringworm in cattle, but may also invade human skin (Ainsworth & Austwick 1973, Ajello & Padhye 1980).
--------------------------------------------------------------------------------
Trichophyton violaceum Bodin
Tinea capitis in both children and adults may be caused by this organism (Ajello & Padhye 1980). The organism is found in the Mediterranean region in particular (Roberts et al. 1984).
--------------------------------------------------------------------------------
Trichosporiella cerebriformis W. Gams
(syn. Sporotrichum cerebriforme de Vries & Kleine-Natrop)
This soil fungus has been isolated from a seborrhoeic-pityriasis-like lesion of the human scalp (Domsch et al. 1980).
--------------------------------------------------------------------------------
Trichosporon beigelii Vuillemin
(syns Trichosporon cutaneum Ota, Pleurococcus beigelii Küchenm. & Rabenh., Trichosporon ovoides Behrend)
This organism, a yeast, is responsible for white piedra, an uncommon infection of the scalp, axillary, and facial hair (Ajello & Padhye 1974, Roberts et al. 1984) and may also occur as an opportunistic pathogen of mucous membranes or skin (Ajello & Padhye 1980, Silva-Hutner & Cooper 1980).
There has been some disagreement in the literature as to whether T. cutaneum or T. beigelii should be considered to be the correct name. do Carmo-Sousa (1970) believes T. cutaneum to be the correct name whereas most medical mycology texts refer to T. beigelii.
--------------------------------------------------------------------------------
Veronaia audouinii Benedek
(syns Microsporum audouinii Gruby, Microsporum langeronii Vanbreuseghem, Microsporum rivalieri Vanbreuseghem)
This organism was formerly a common agent of epidemic tinea capitis in young children (Ajello & Padhye 1980).
--------------------------------------------------------------------------------
Volutella cinerescens
This organism has been isolated from keratitis lesions (Rebell & Forster 1980).
--------------------------------------------------------------------------------
Wangiella dermatitidis McGinnis
(syns Hormiscium dermatitidis Kano, Phialophora dermatitidis Emmons, Exophiala dermatitidis De Hoog)
This organism is an important aetiological agent of chromoblastomycosis and of phaeohyphomycosis (Ajello et al. 1974, McGinnis 1978, Roberts et al. 1984). The fungus has most frequently been found in patients in Japan (McGinnis 1980, 1983). See also Phialophora Medlar.
--------------------------------------------------------------------------------
Zygosporium masonii Hughes
Minato et al. (1973) investigated the cytotoxicity and skin irritancy of a number of natural and semi-synthetic cytochalasins (or zygosporins) derived from the culture filtrate of this fungus. The two properties were found not to be directly related to one another; the most potent irritants amongst the natural cytochalasins were zygosporins A and D.
========================================================================
11.) FUNGI - ENTOMOPHTHORACEAE
========================================================================
This family is classifed in the subdivision Zygomycotina. It comprises about 167 species in 12 genera (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Basidiobolus haptosporus Drechsler
(syn. Basidiobolus meristosporus Drechsler)
This organism can produce a subcutaneous zygomycosis known as basidiobolomycosis. The disease usually occurs in children and is mainly seen in East Africa although cases elsewhere in the tropics have been reported (Tio et al. 1966, Greer & Friedman 1966, Roberts et al. 1984).
--------------------------------------------------------------------------------
Conidiobolus coronatus Batko
(syns Entomophthora coronata Kevork., Conidiobolus coronatus Sriniv. & Thirum., Delacroixia coronata Kjøller)
This organism is typically associated with decaying insect remains in the soil (Talbot 1971).
Rhinoentomophthoromycosis, a zygomycosis also known as conidiobolomycosis, is produced by this organism. The disease is normally confined to the face, usually originating from the inferior turbinates of the nose from where it spreads slowly and progressively (Roberts et al. 1984). The disease occurs in the tropics (Greer 1980, Domsch et al. 1980).
In a case described by Herstoff et al. (1978), the patient failed to respond with an inflammatory reaction when challenged with croton oil (see Croton tiglium L., fam. Euphorbiaceae). This observation is intriguing but difficult to interpret since no further information was provided regarding mode of application and effects on control subjects.
========================================================================
12.) FUNGI - GOMPHACEAE
========================================================================
Ramaria flava Quélet
(syn. Clavaria flava Fr.)
Fairy Clubs
Two cases of cutaneous sensitisation to edible mushrooms (Boletus edulis Bull. ex Fr. and B. luteus L. ex Fr., fam. Boletaceae; Lactarius deliciosus Fr., fam. Russulaceae; and Clavaria flava) were reported. In one case hypersensitivity was also demonstrated after eating the mushrooms in question, fried. Boiling seems to destroy the antigenic effect of mushrooms. From a practical point of view, it is important to be familiar with the cases, as for example in differentiating between similar eruptions caused by sunlight or hypersensitivity to autumn flowers (Hellerström 1941).
========================================================================
13.) FUNGI - GYMNOASCACEAE
========================================================================
This family of ascomycetes comprises 100 species in 32 genera (Hawksworth et al. 1983).
Ajellomyces dermatitidis McDonough & Lewis
Anamorph: Blastomyces dermatitides Gilchrist & Stokes
This organism can cause blastomycosis, also known as Gilchrist's disease, a systemic/cutaneous mycosis (Ainsworth & Austwick 1973, Larsh & Goodman 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Arthroderma Berk.
About 15 species of Arthroderma are known, all of which are keratophilic. These fungi commonly occur near animal burrows and bird nests, but a number are parasitic and hence pathogenic to man and other mammals causing the diseases known as ringworm.
These organisms are better known as their anamorphs of the form-genus Trichophyton Malmsten. In fact, most of the pathogenic Trichophyton species are considered under subdivision Deuteromycotina since their teleomorphs are as yet unknown.
--------------------------------------------------------------------------------
Arthroderma benhamiae Ajello & Cheng
Anamorph: Trichophyton mentagrophytes Blanchard
(syns Trichophyton asteroides Sabouraud, Trichophyton granulosum, Trichophyton gypseum Bodin, Trichophyton interdigitale Priestley, Epidermophyton interdigitale Kesteven, Trichophyton quinckeanum MacLeod & Munde)
This organism is a common cause of ringworm in humans and animals (Ainsworth & Austwick 1973, Ajello & Padhye 1980, Roberts et al. 1984). It can also produce a vesicular form of athlete's foot (Sande & Mandell 1985).
--------------------------------------------------------------------------------
Arthroderma simii Stockdale, Mackenzie, & Austwick
(syn. Epidermophyton simii Pinoy)
Anamorph: Trichophyton simii Stockdale, Mackenzie, & Austwick
This organism may cause ringworm in various animals and man (Ainsworth & Austwick 1973).
--------------------------------------------------------------------------------
Arthroderma uncinatum Dawson & Gentles
Anamorph: Trichophyton ajelloi Ajello
(syn. Keratinomyces ajelloi Vanbreuseghem)
Skin infections caused by this organism have been observed rarely in animals and in isolated cases in man (Ainsworth & Austwick 1973, Domsch et al. 1980).
--------------------------------------------------------------------------------
Emmonsiella capsulata Kwon-Chung
Anamorphs: Histoplasma capsulatum Darling var. capsulatum, Histoplasma capsulatum Darling var. duboisii Cif.
(syn. Histoplasma duboisii Vanbreuseghem)
The spores of these organisms can cause the lung disease histoplasmosis if inhaled (Roberts et al. 1984). Most patients develop an hypersensitivity state that is readily demonstrated by skin tests (Larsh & Goodman 1980).
The disease may exhibit cutaneous symptoms such as erythema nodosum and multiforme. African histoplasmosis caused by H. capsulatum var. duboisii may present with painless nodules, abscesses, or ulcers, or acneiform papules (Roberts et al. 1984).
--------------------------------------------------------------------------------
Nannizzia Stockdale
This genus of ascomycetes is closely related to Arthroderma Berk. and is similarly capable of producing the disease known as ringworm. Generally, these organisms are only infrequently found to be responsible for human ringworm infections, being more commonly found affecting livestock (Ainsworth & Austwick 1973, Ajello & Padhye 1980).
The organisms are better known as their anamorphs in the form-genus Microsporum Gruby, under which heading some are considered because their teleomorphs are as yet unknown.
The following species have been reported as causative organisms in human ringworm infections (Ainsworth & Austwick 1973, Domsch et al. 1980, Ajello & Padhye 1980):
N. cajetani Ajello
Anamorph: Microsporum cookei Ajello
N. fulva Stockdale
Anamorph: Microsporum fulvum Uriburu
N. grubyia Georg, Ajello, Friedman, & Brinkman
Anamorph: Microsporum vanbreuseghemii George, Ajello, Friedman, & Brinkman
N. gypsea Stockdale
Anamorph: Microsporum gypseum Guiart & Grigorakis
N. obtusa Dawson & Gentles
Anamorph: Microsporum nanum Fuentes
N. persicolor Stockdale
Anamorph: Microsporum persicolor Guiart & Grigorakis
(syn. Trichophyton persicolor Sabouraud)
========================================================================
14.) FUNGI - HELVELLACEAE
========================================================================
This family of ascomycetes comprises 60 species in 6 genera (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Gyromitra esculenta Fr.
Lorchel, Turban Fungus
The skin and the eyes may be irritated by handling the fungus (North 1967).
When damaged, the fungus releases monomethylhydrazine and methylformylhydrazine from stored hydrazones of ethanal (= gyromitrin), pentanal, and hexanal (Chilton 1978).
========================================================================
15.) FUNGI - HYPOCREACEAE
========================================================================
This is a family of about 76 genera and 520 species classified in the subdivision Ascomycotina. The family is also known as the Nectriaceae. These organisms are of cosmopolitan distribution; many are plant parasites (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Gibberella moniliformis Winel
Anamorph: Fusarium moniliforme Sheldon
This organism may cause mycotic ulcers of the cornea (Rebell & Forster 1974) and mycetomas (Padhye & Ajello 1980).
--------------------------------------------------------------------------------
Nectria Fr.
The genus Nectria Fr. is classified by some authorities in the family Hypocreaceae. Anamorphs are found in the form-genera Acremonium Link ex Fr., Fusarium Link ex Fr., Verticillium Nees ex Link, Myrothecium Tode ex Fr., and many others (Domsch et al. 1980).
--------------------------------------------------------------------------------
Nectria haematococca Berk. & Br. var. brevicona Gerlach
Anamorph: Fusarium solani Sacc.
(syn. Fusisporium solani Mart.)
This organism is the most important cause of mycotic ulcers of the cornea (Rebell & Forster 1974). It may also cause mycetomas (Padhye & Ajello 1980).
--------------------------------------------------------------------------------
Nectria haematococca Berk. & Br. var. haematococca
(syn. Hypomyces haematococcus Wollenw.)
Anamorph: Fusarium eumartii Carp.
Mycelial extracts of this organism caused toxic reactions on the skin of rabbits (Domsch et al. 1980).
--------------------------------------------------------------------------------
Nectria inventa Pethybr.
Anamorph: Verticillium tenereum Link
N. inventa was isolated from a keratomycosis in one of 88 cases (Domsch et al. 1980).
========================================================================
16.) FUNGI - MICROASCACEAE
========================================================================
This family of ascomycetes, also known as the Lophotrichaceae, comprises some 9 genera and 38 species found in soil and dung (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Pseudallescheria boydii McGinnis, Padhye, & Ajello
(syns Petriellidium boydii Malloch, Allescheria boydii Shear)
Anamorph: Scedosporium apiospermum auct.
(syns Monosporium apiospermum Sacc., Aleurisma apiospermum Maire)
"White grain" mycetomas, maduromycosis (madura foot), and other fungal infections produced by this organism have been reported from Australia and West Africa (Domsch et al. 1980, Padhye & Ajello 1980, Roberts et al. 1984).
========================================================================
17.) FUNGI - MORCHELLACEAE
========================================================================
This small family of ascomycetes comprises 25 species in 5 genera (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Morchella esculenta St Amans
Common Morel
Industrial workers preserving this edible fungus developed keratoconjunctivitis and respiratory irritation (Pick 1927, Grant 1974).
========================================================================
18.) FUNGI - MUCORACEAE
========================================================================
This family of 119 species in 21 genera is classified in the subdivision Zygomycotina (Hawksworth et al. 1983).
A systemic zygomycosis known as mucormycosis is known as a rare opportunistic infection in immunosuppressed or diabetic patients. Wounds covered with dressings that are contaminated with these organisms may also become infected. Mucormycosis may be caused by several species of the genera Absidia van Tieghem, Mucor Mich. ex St.-Am., and Rhizopus Ehrenb. (Greer 1980, Roberts et al. 1984).
--------------------------------------------------------------------------------
Absidia corymbifera Sacc. & Trotter
(syns Absidia cornealis Dodge, Absidia italiana Dodge, Absidia ramosa Lendner, Mucor corymbifer F. Cohn)
This species has been recorded as causing mucormycosis (Ajello et al. 1976).
--------------------------------------------------------------------------------
Mucor Mich. ex St.-Am.
The following species have been recorded as causing mucormycosis (Ajello et al. 1976):
M. pusillus Lindt
(syn Mucor miehei Cooney & Emerson)
M. ramosissimus Samsutsewitsch
--------------------------------------------------------------------------------
Mucor mucedo Mich. ex St.-Am.
Various moulds have been suspected as causes of dermatitis, including Mucor mucedo (White 1934).
--------------------------------------------------------------------------------
Mucor ramannianus Moller
This organism yields ramycin, an antibiotic that is identical with the better known fusidic acid (Hawksworth et al. 1983). See Fusidium coccineum Fuckel, subdivision Deuteromycotina.
--------------------------------------------------------------------------------
Rhizopus Ehrenb.
The following species have been recorded as causing mucormycosis (Ajello et al. 1976):
R. arrhizus Fischer
R. microsporus van Tieghem
R. oryzae Went & Prinsen Geerligs
R. rhizopodiformis Zopf
(syns Rhizopus chinensis Saito, Rhizopus cohnii Berlese & De Toni, Mucor rhizopodiformis F. Cohn)
--------------------------------------------------------------------------------
Rhizopus stolonifer Lind.
(syn. Rhizopus nigricans Ehrenb. ex Corda)
White (1934) lists Rhizopus nigricans as a mould that has been suspected as a cause of dermatitis.
Ajello et al. (1976) reviewed several cases of zygomycosis that had been attributed to this organism but doubted the identifications made, especially since the organism is incapable of growing at 37°C.
========================================================================
19.) FUNGI - PHAEOSPHAERIACEAE
========================================================================
The family is classified in the subdivision Ascomycotina (Hawksworth et al. 1983).
-------------------------------------------------------------------------------
Leptosphaeria Ces. & de Not.
About 100 species are recognised in this genus; the organisms occur widely as plant pathogens. Their family position is not clear, some authorities regarding the genus as belonging to the Pleosporaceae (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Leptosphaeria senegalensis Baylet, Camain, & Segretain
Leptosphaeria tompkinsii El-Ani
Cases of mycetoma caused by these organisms have been reported from northern tropical West Africa (Padhye & Ajello 1980).
========================================================================
20.) FUNGI - PIEDRAIACEAE
========================================================================
The family is classified in the subdivision Ascomycotina (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Piedraia hortae Fonseca & Leão
Anamorph: Trichosporon hortae Brumpt
This organism is responsible for black piedra, a fungus infection of scalp (and occasionally axillary and pubic) hair which occurs primarily in tropical areas of the world (Ajello & Padye 1980, Roberts et al. 1984).
========================================================================
21.) FUNGI - PUCCINIACEAE
========================================================================
This family of "rust" fungi is classified in the order Uredinales of the subdivision Basidiomycotina. Rusts are of great importance as pathogens of higher plants, especially grasses and cereals.
--------------------------------------------------------------------------------
Puccinia Pers. ex Pers.
Smuts and rusts have been suspected of producing skin irritation, stomatitis, and inflammation of mucous membranes (Pammel 1911, Hurst 1942). Pammel (1911) noted that considerable irritation of the nose and throat is experienced when cereals infected with rusts are threshed. See also Ustilago Roussel, fam. Ustilaginaceae and Coleosporium Lév., fam. Coleosporiaceae.
--------------------------------------------------------------------------------
Puccinia graminis Pers.
Wheat Rust
The fungus can attack several species of grasses and cereals, including wheat (Triticum aestivum L., fam. Gramineae). Wheat rust has been reported to cause dermatitis (Schwartz et al. 1957) as well as asthma (Cadham 1924).
--------------------------------------------------------------------------------
Puccinia coronata Corda
This species can cause contact dermatitis (Leonardi 1954).
========================================================================
21.) FUNGI - PYRENOPHORACEAE
========================================================================
The family is classified in the subdivision Ascomycotina (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Cochliobolus Drechsler
The anamorphic form-genus Curvularia Boedijn, which comprises some 35 species of mostly tropical and subtropical facultative plant parasites, has been identified with the teleomorphic genus Cochliobolus (Domsch et al. 1980). It is considered by some authorities to be a member of the family Pleosporaceae (Hawksworth et al. 1983). See also Curvularia Boedijn, subdivision Deuteromycotina.
--------------------------------------------------------------------------------
Cochliobolus geniculatus R. Nelson
(syn. Pseudocochliobolus geniculatus Tsuda et al.)
Anamorph: Curvularia geniculata Boedijn
(syn. Helminthosporium geniculatum Tracy & Earle)
Cochliobolus lunatus R. Nelson & Haasis
(syn. Pseudocochliobolus lunatus Tsuda et al.)
Anamorph: Curvularia lunata Boedijn
(syn. Helminthosporium curvulum Sacc.)
These two species have been reported to cause mycetomas. Curvularia geniculata has affected dogs in the U.S.A. whilst Curvularia lunata has affected humans in Senegal and Sudan (Padhye & Ajello 1980).
--------------------------------------------------------------------------------
Cochliobolus spicifera R. Nelson
Anamorph: Curvularia spicifera Boedijn
(syns Drechslera spicifera v. Arx, Helminthosporium spiciferum Nicot, Brachycladium spiciferum Bainier)
McGinnis (1980) notes that this species is a recognised pathogen.
========================================================================
22.) FUNGI - RUSSULACEAE
========================================================================
Lactarius deliciosus S.F. Gray
Saffron Milk Cap, Orange Agaric
A case of cutaneous sensitisation to the edible mushrooms Boletus luteus L. ex Fr., fam. Boletaceae; Lactarius deliciosus Fr., and Ramaria flava Quél., fam. Gomphaceae was reported. Hypersensitivity was also demonstrated after eating the mushrooms in question, fried. Boiling seems to destroy the antigenic effect of mushrooms. From a practical point of view, it is important to be familiar with the cases, as for example in differentiating between similar eruptions caused by sunlight or hypersensitivity to autumn flowers (Hellerström 1941).
========================================================================
23.) FUNGI - SACCHAROMYCETACEAE
========================================================================
This family of some 240 species of yeast fungi in 37 genera is classified in the subdivision Ascomycotina. It may also be named the Saccharomycodaceae or the Schizosaccharomycetaceae in some publications (Hawksworth et al. 1983). The family includes the commercially important fermentative yeasts used by brewers, bakers, and wine-makers.
--------------------------------------------------------------------------------
Pichia guilliermondii Wickerham
(syn. Saccharomyces krusei Castellani)
Anamorph: Candida guilliermondii Langeron & Guerra
(syn. Endomyces guilliermondii Castellani)
This organism may be found as an opportunistic pathogen in man (Roberts et al. 1984).
--------------------------------------------------------------------------------
Saccharomyces cerevisiae Meyen ex E. Hansen
Baker's Yeast
This yeast, which is used in baking, brewing, and wine making, has been reported to cause dermatitis. Brewery workers who scrape off masses of yeasts adhering to fermentation casks with their finger-nails develop crusted excrescences beneath the nails and destruction of the nail-plates (White 1934).
This organism is responsible for occasional cases of thrush and vulvovaginitis (Silva-Hutner & Cooper 1980).
========================================================================
24.) FUNGI - SAKSENAEACEAE
========================================================================
This family of 2 species in 2 genera is classified in the subdivision Zygomycotina (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Saksenaea vasiformis Saksena
Ajello et al. (1976) described a case of zygomycosis caused by this organism in cranial and facial wounds sustained in an automobile accident.
========================================================================
25.) FUNGI - SCHIZOPHYLLACEAE
========================================================================
This family comprises some 50 species of lignicolous fungi in 4 genera (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Schizophyllum commune Fr.
This organism may cause a subcutaneous infection described as a hyalohyphomycosis (McGinnis et al. 1985). Kligman (1950) investigated a case of onychomycosis associated with an itching rash involving the large toes of both feet in a 30 year old male and concluded that it was probably caused byS. commune.
========================================================================
26.) FUNGI - SCLEROTINIACEAE
========================================================================
This family, which has been divided into 28 genera and 245 species (Hawksworth et al. 1983), is classified in the subdivision Ascomycotina. It is an important family from an economic point of view since its members are responsible for much damage to fruit, vegetables, and ornamental plants.
The grey mould Botrytis cinerea Pers. ex Nocca & Balb. is a particularly well known organism which is in fact a form-species complex connected to several teleomorphs classified in the Sclerotiniaceae. These include Botryotinia fuckeliana Whetzel, Botryotinia convoluta Whetzel, Sclerotinia draytonii Buddin & Wakef., Botryotinia pelargonii Røed, and others (Domsch et al. 1980).
--------------------------------------------------------------------------------
Sclerotinia sclerotiorum de Bary
(syn. Peziza sclerotiorum Lib.)
Pink Rot
Celery (Apium graveolens L., fam. Umbelliferae) infected with this fungus contains the phototoxic furocoumarins bergapten and xanthotoxin, and can therefore elicit photodermatitis (see Umbelliferae). Austad & Kavli (1983) reported the occurrence of phototoxic contact dermatitis of the hands and forearms of 11 celery harvesters in Norway. The conditions favouring an epidemic include diseased celery, a sunny day following wet weather, and unprotected harvesters.
========================================================================
27.) FUNGI - TRICHOCOMACEAE
========================================================================
This family of ascomycetes comprises 120 species in 24 genera (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Emericella nidulans Vuillemin
Anamorph: Aspergillus nidulans Winter
Cases of "white grain" mycetomas caused by this organism have been described from Senegal, Sudan, and Tunisia (Padhye & Ajello 1980, Roberts et al. 1984). Onchomycosis and mandibular periostitis associated with this organism have also been described (Domsch et al. 1980).
========================================================================
28.) FUNGI - USTILAGINACEAE
========================================================================
This family of "smut" fungi is classified in the subdivision Basidiomycotina. It includes a number of important crop plant pathogens.
--------------------------------------------------------------------------------
Filobasidiella neoformans Kwon-Chung
(syn. Saccharomyces neoformans Sanfelice)
Anamorph: Cryptococcus neoformans Vuillemin
(syn. Torulopsis neoformans Redaelli)
This organism is best known as its anamorphic form, the teleomorph having only recently (Kwon-Chung 1976) been described. It lives naturally on soil contaminated with bird droppings. The organism can produce a diffuse pulmonary infection if inhaled, leading to meningitis and followed by localised abscesses or granulomas (cryptococcoma or toruloma) in the lungs, lymph nodes, bones, and skin (Silva-Hutner & Cooper 1980). The disease is known as cryptococcosis. Skin involvement may occur in 10-15% of cases (Roberts et al. 1984). An hypersensitivity state has been demonstrated in some individuals suffering from the disease (Larsh & Goodman 1980).
--------------------------------------------------------------------------------
Ustilago Roussel
The genus Ustilago is one of a number of genera of fungi known as smuts which are classified in the order Ustilaginales. Two families of smut fungi are recognised, namely Tilletiaceae and Ustilaginaceae (Talbot 1971).
A disorder resembling acrodynia occurs following ingestion of the corn-smut Ustilago (Watt & Breyer-Brandwijk 1962).
Smuts and rusts have been suspected of producing skin irritation, stomatitis, and inflammation of mucous membranes (Hurst 1942). See also Coleosporium Lév., fam. Coleosporiaceae and Puccinia Pers. ex Pers., fam. Pucciniaceae. Irritant properties have been ascribed to the following species (Pammel 1911, Barjaktarovic & Bogdanovic 1933, Preininger 1937/38):
U. avenae Rostrup
(syn. Ustilago avenae Jensen)
U. jensenii Rostrup
(syn. Ustilago hordei Lagerh.)
U. neglecta Neissl
U. nuda Rostrup
(syns Ustilago nuda Kell. & Swingle, Ustilago tritici Rostrup, Ustilago tritici Jensen)
U. utriculosa Tul.
U. zeae Unger
(syn. Ustilago maydis Corda)
--------------------------------------------------------------------------------
Ustilago bromivora A. Fisch. v. Waldh.
This smut, which frequently affects the prairie grass Bromus unioloides Kunth (syn. Bromus catharticus Vahl), has been reported to cause severe hay fever and to raise wheals on any part of the body that may be scratched. One smut body in a glass of water produced a solution that gave rise to large wheals when applied to broken skin (Cleland 1943).
--------------------------------------------------------------------------------
Ustilago hypodytes Fr.
This species was incriminated in reed dermatitis (Arundo donax L., fam. Gramineae); positive epicutaneous tests to the fungus were observed in rabbits (Gerbaud 1885). This represents an early use of the epicutaneous test.
========================================================================
29.) FUNGI - ZOPFIACEAE
========================================================================
This family now includes the Testudinaceae. It is a member of the largest and most varied group of ascomycetes known as the Dothideales (Hawksworth et al. 1983).
--------------------------------------------------------------------------------
Neotestudina rosatii Segretain & Destombes
(syn. Zopfia rosatii D. Hawksworth & C. Booth)
This organism was originally isolated from severe "white grain" mycetomas in man in Somalia, other African countries, and in Australia (Domsch et al. 1980, Padhye & Ajello 1980).
========================================================================
30.) DERMATOLOGY ASPECTS
========================================================================
The airborne spores of a number of moulds are known to have a causal role in many cases of atopic and seasonal eczemas, rhinitis, and asthma. Most commonly implicated are species of Alternaria Nees ex Fr., Aspergillus Mich. ex Fr., and Penicillium Link ex Fr. (Feinberg 1939, Tuft et al. 1950, Bocobo et al. 1954, Gomez-Orbanaja & Quinones-Caravia 1953, Jillson & Adami 1955, Storck 1955, Jillson 1957, Strauss & Kligman 1957, Prince et al. 1960, Ofuji et al. 1961, Rajka 1963, Watanabe & Fujisawa 1965, Fujisawa et al. 1966). Crude extracts of moulds produced eczematous changes by patch test and also by inhalation. Type I hypersensitivity reactions can occur from inhalation of fungi (Bruce 1963).
========================================================================
31.) VETERINARY ASPECTS
========================================================================
As in man, fungi may affect animals either by contact (including subcutaneous inoculation), by ingestion, or by inhalation. Many of the pathogenic fungi can produce the same diseases in both humans and livestock. Interested readers are referred to specialised texts on the topic such as that produced by Ainsworth & Austwick (1973).
Perhaps most commonly encountered are ringworm infections which are caused by members of the form-genera Microsporum Gruby, Trichophyton Malmst., and Epidermophyton Sabouraud. Exposure to the spores of, and ingestion of grass contaminated with the deuteromycete Pithomyces chartarum M.B. Ellis (syns Sporidesmium chartarum Berk. & Curt., Sporidesmium bakeri Syd.) causes facial eczema in sheep and cattle, photosensitisation of the skin, liver lesions, and a raised phylloerythrin concentration in the blood. These toxic effects are attributable to the sporidesmins produced by the organism (Russell 1960, Leach & Tulloch 1971, Ainsworth & Austwick 1973, Domsch et al. 1980). Consumption of hay contaminated with the deuteromycete Stachybotrys chartarum Hughes (syn. Stachybotrys alternans Bonord.) produces a disease in horses known as stachybotryotoxicosis. It is characterised by ulceration in the nose, mouth, throat, and gastrointestinal tract (Brian et al. 1947, Domsch et al. 1980).
Alternaria alternata Keissler (syn. A. tenuis Nees) and other Alternaria Nees ex Fr. species are thought to cause allergic eczema in dogs and horses (Allen 1945, Austwick 1966). Pammel (1911) described the production of inflammation of skin and mucous membranes in animals after contact with the "rape fungus" (Alternaria exitiosa Jørstad, syn. Sporidesmium exitiosum Kühn) which may infect brassicas (fam. Cruciferae).
Sporotrichosis (see Sporothrix schenkii Hektoen & Perkins, subdivision Deuteromycotina), histoplasmosis (see Emmonsiella capsulata Kwon-Chung, fam. Gymnoascaceae), blastomycosis (see Ajellomyces dermatitidis McDonough & Lewis, fam. Gymnoascaceae), candidiasis (see Candida albicans Berkhout, subdivision Deuteromycotina) and other fungal diseases known in man are also found in livestock. Diseases more typically associated with animals include the respiratory diseases adiaspiromycosis caused by Emmonsia parva Ciferri & Montemartini (syn. Haplosporangium parvum Emmons & Ashburn) and Emmonsia crescens Emmons & Jellison; rhinosporidiosis caused by Rhinosporidium seeberi Seeber; and equine nasal granuloma caused by Conidiobolus coronatus Batko, fam. Entomophthoraceae. Miscellaneous fungal infections of animals include epizootic lymphangitis caused by Histoplasma farciminosum Redaelli & Ciferri (syn. Cryptococcus farciminosum Rivolta), and a hyphomycosis produced by Hyphomyces destruens, a fungus of uncertain taxonomic position (Ainsworth & Austwick 1973).
A number of organisms are capable of producing diseases such as mycotic mastitis and mycotic abortion (Ainsworth & Austwick 1973).
========================================================================
32.) REFERENCES
========================================================================
1.) Ainsworth GC and Austwick PKC (1973) Fungal Diseases of Animals, 2nd edn. Farnham Royal, England: Commonwealth Agricultural Bureaux.
2.) Ajello (1968)
3.) Ajello L and Padhye A (1980) Dermatophytes and the agents of superficial mycoses. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 541. Washington, D.C.: American Society for Microbiology.
4.) Ajello L, Georg LK, Steigbigel RT and Wang CJK (1974) A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66(3): 490-498.
5.) Ajello L, Dean DF and Irwin RS (1976) The zygomycete Saksenaea vasiformis as a pathogen of humans with a critical review of the etiology of zygomycosis. Mycologia 68(1): 52-62.
6.) Allen T (1945) Some observations on the relationship of Alternaria tenuis to canine eczema. Journal of the American Veterinary Medical Association 106: 163.
7.) Austad J and Kavli G (1983) Phototoxic dermatitis caused by celery infected by Sclerotinia sclerotiorum. Contact Dermatitis 9: 448-451.
8.) Austwick PKC (1966) The role of spores in the allergies and mycoses of man and animals. In: Madelin MF (Ed.) The Fungus Spore. pp. 321. London: Butterworths.
9.) Austwick PKC and Longbottom JL (1980) Medically important Aspergillus species. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 620. Washington, D.C.: American Society for Microbiology.
10.) Barjaktarovic SS and Bogdanovic SB (1933) Untersuchungen über die Wirkung des Maisbrandes (Ustilago maidis). Arch. Exp. Path. Pharmak. 173: 381.
11.) Benham RW (1947) Biology of Pityrosporum ovale. In: Nickerson WJ (Ed.) Biology of Pathogenic Fungi. pp. 63. Waltham, Mass.: Chronica Botanica Co.
12.) Bocobo FC, Curtis AC, Block WD and Stubbart FJ (1954) Studies on fungi encountered in the atmosphere. II. Production of dermatitis in guinea pigs by crude ether-soluble extracts of Alternaria, Hormodendrum, Penicillium and Aspergillus. Journal of Investigative Dermatology 23(6): 489-496.
13.) Bowden JP and Schantz EJ (1955) The isolation and characterization of dermatitic compounds produced by Myrothecium verrucaria. J. Biol. Chem. 214: 365-372.
14.) Brian PW, Curtis PJ and Hemming HG (1947) Glutinosin: a fungistatic metabolic product of the mould Metarrhizium glutinosum S. Pope. Proc. R. Soc., Ser. B 135: 106-132.
15.) Brian PW, Hemming HG and Jefferys EG (1948) Production of antibiotics by species of Myrothecium. Mycologia 40(3): 363-368.
16.) Bruce RA (1963) Bronchial and skin sensitivity in asthma. International Archives of Allergy 22: 294.
17.) Cadham FT (1924) Asthma due to grain rusts. Journal of the American Medical Association 83: 27.
18.) Campbell & White (1989)
19.) Campbell CK et al. (1973) Fungal infection of skin and nails by Hendersonula toruloidea. British Journal of Dermatology 89: 45.
20.) Chilton WS (1978) Chemistry and mode of action of mushroom toxins. In: Rumack BH and Salzman E (Eds) Mushroom Poisoning: Diagnosis and Treatment. pp. 87. Florida: CRC Press, Inc.
21.) Cleland JB (1943) Plants, including fungi, poisonous or otherwise injurious to man in Australia. IV. Medical Journal of Australia ii: 161.
22.) Cooper BH (1985) Taxonomy, classification, and nomenclature of fungi. In: Lennette EH, Balows A, Hausler WJ and Shadomy HJ (Eds) Manual of Clinical Microbiology, 4th edn. pp. 495-499. Washington, D.C.: American Society for Microbiology.
23.) Cronin E (1980) Contact Dermatitis. Edinburgh: Churchill Livingstone.
24.) Deighton FC and Mulder JL (1977) Mycocentrospora acerina as a human pathogen. Trans. Br. Mycol. Soc. 69: 326.
25.) do Carmo-Sousa L (1970) Genus 11. TRICHOSPORON Behrend. In: Lodder L (Ed.) The Yeasts. A taxonomic study, 2nd edn. pp. 1309. Amsterdam: North-Holland Publishing Co.
26.) Domsch KH et al. (1980) Compendium of Soil Fungi. Vol. 1 & 2. London: Academic Press.
27.) Emmons CW, Joe L-K, Tjoei Eng N-I, Pohan A, Kertopati S and van der Meulen A (1957) Basidiobolus and Cercospora from human infections. Mycologia 49(1): 1-10.
28.) Feinberg SM (1939) Seasonal atopic dermatitis. The role of inhalant atopens. Archives of Dermatology and Syphilology 40: 200.
29.) Frankland AW and Hay MJ (1951) Dry rot as a cause of allergic complaints. Acta Allerg. IV: 186. Cited by Rajka (1963)
30.) Fujisawa S et al. (1966) Eczematous dermatitis produced by air borne molds. Archives of Dermatology 94: 413.
31.) Gerbaud (1885) Ann. Derm. Syph. Cited by White (1887)
32.) Gomez-Orbanaja J and Quinones-Caravia PA (1953) La alergia a inhalantes (hongos) en el eczema de las manos. Acta Dermosifilogr. 44: 610. Cited in Z. Haut- u. GeschlKrankh. 89: 289 (1954)
33.) Grant WM (1974) Toxicology of the Eye, 2nd edn. Springfield, Ill.: CC Thomas.
34.) Greer DL (1980) Agents of zygomycosis (phycomycosis). In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 611. Washington, D.C.: American Society for Microbiology.
35.) Greer DL and Friedman L (1966) Studies on the genus Basidiobolus with reclassification of the species pathogenic to man. Sabouraudia 4: 231.
36.) Grimes GL (1978) Principles of mushroom identification. In: Rumack BH and Salzman E (Eds) Mushroom Poisoning: Diagnosis and Treatment. pp. 3. Florida: CRC Press Inc.
37.) Hammerschmidt DE (1980) Szechwan purpura. New England Journal of Medicine 302: 1191.
38.) Hawksworth DL et al. (1983) Ainsworth & Bisby's Dictionary of the Fungi, 7th edn. Kew: Commonwealth Mycological Institute.
39.) Hellerström S (1941) Sensitization to edible mushrooms. Acta Dermato-Venereologica 22: 331.
40.) Herstoff JK et al. (1978) Rhinophycomycosis entomophthorae. Archives of Dermatology 114: 1674.
41.) Holmes S (1983) Outline of Plant Classification. London: Longman Group Ltd.
42.) Hopkins HH (1952) Mushroom dermatitis. Md St. Med. J. 1: 504.
43.) Hopkins HH (1953) Mushroom dermatitis. Report of a case. AMA Archives of Dermatology and Syphilology 67: 632.
44.) Hurst E (1942) The Poison Plants of New South Wales. Sydney: N.S.W. Poison Plants Committee.
45.) Jillson OF (1957) Allergic dermatitis produced by pathogenic and saprophytic fungi. Annals of Allergy 15: 14.
46.) Jillson OF and Adami M (1955) Allergic dermatitis produced by inhalant molds. AMA Archives of Dermatology 72: 411.
47.) Joseph R et al. (1965) A propos de deux observations d'allergie à Candida albicans chez l'enfant, associante à des manifestations articulaires des troubles respiratoires, digestifs et cutanés. Revue Fr. Allerg. 5: 37.
48.) Kaufman L (1980) Serodiagnosis of fungal diseases. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 628. Washington, D.C.: American Society for Microbiology.
49.) Kligman AM (1950) A basidiomycete probably causing onychomycosis. Journal of Investigative Dermatology 14(1): 67-70.
50.) Kwon-Chung KJ (1976) A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68(4): 942-946.
51.) Larsh HW and Goodman NL (1980) Fungi of systemic mycoses. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 577. Washington, D.C.: American Society for Microbiology.
52.) Laverde S et al. (1973) Mycotic keratitis; 5 cases caused by unusual fungi. Sabouraudia 11: 119.
53.) Leach CM and Tulloch M (1971) Pithomyces chartarum, a mycotoxin-producing fungus, isolated from seed and fruit in Oregon. Mycologia 63(5): 1086-1089.
54.) Leonardi G (1954) Spore di lichene boschivo (Palmeria caperata) causa di allergosi cutanea. Folia Allerg. 1: 219.
55.) Mackinnon JE et al. (1949) Investigaciones sobre las maduromicosis y sus agentes. An. Fac. Med. Univ. Montevideo 34: 231. Cited by Neuhauser (1955)
56.) Mandoul R et al. (1954) La maladie de la canne de Provence en Algérie. Bull. Soc. Path. Exot. 47: 572.
57.) Martin H (Ed.) (1969) Insecticide and Fungicide Handbook, 3rd edn. Oxford: Blackwell Scientific.
58.) McCombs RP (1972) Diseases due to immunologic reactions in the lungs. New England Journal of Medicine 286: 1186 & 1245.
60.) McGinnis MR (1978) Human pathogenic species of Exophiala, Phialophora, and Wangiella. Scient. Publs Pan-Am. Hlth Org. 356: 37.
61.) McGinnis MR (1979) Taxonomy of Exophiala werneckii and its relationship to Microsporum mansonii. Sabouraudia 17: 145.
62.) McGinnis MR (1980) Dematiaceous fungi. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 602. Washington, D.C.: American Society for Microbiology.
63.) McGinnis MR (1983) Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. Journal of the American Academy of Dermatology 8: 1.
64.) McGinnis MR et al. (1985) Mycotic diseases. A proposed nomenclature. Int. J. Derm. 24: 9.
65.) Mehregan AH and Rudner EF (1980) Implantation dermatosis: wood splinter with fungus contamination. Journal of Cutaneous Pathology 7: 330.
66.) Minato H, Katayama T, Matsumoto, Katagiri K, Matsuura S, Sunagawa N, et al. (1973) Structure-activity relationships among zygosporin derivatives. Chemical and Pharmaceutical Bulletin 21(10): 2268-2277.
67.) North PM (1967) Poisonous Plants and Fungi. London: Blandford Press.
68.) Ofuji S et al. (1961) Studies on the role of airborne fungi in several skin diseases. 2. Studies on the seasonal variations of the mould counts in the Kyoto area. Acta Derm., Kyoto 56: 205. Cited by Fujisawa et al. (1966)
69.) Padhye AA and Ajello L (1980) Fungi causing eumycotic mycetomas. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 595. Washington, D.C.: American Society for Microbiology.
70.) Pammel LH (1911) A Manual of Poisonous Plants. Cedar Rapids, Iowa: Torch Press.
71.) Panzani R (1962) Étude de l'allergie entre la graine de ricin et spondylocladium. Int. Archs Allergy Appl. Immun. 21: 288.
72.) Pick L (1927) Augen- und Schleimhauterkrankungen durch Morchelausdunstungen (gewerbliche Massenerkrankung). Z. Augenheilk. 61: 325.
73.) Preininger T (1937/8) Durch Maisbrand (Ustilago maydis) bedingte Dermatomykose. Arch. Derm. Syph. 176: 109.
74.) Prince HE et al. (1960) The importance of inhalant mold sensitivity in atopic dermatitis. Annals of Allergy 18: 186.
75.) Rajka G (1963) Studies in hypersensitivity to molds and staphylococci in prurigo Besnier (atopic dermatitis). Acta Dermato-Venereologica 43(Suppl. 54): 1-139.
76.) Ramirez MA (1930) Trichophytin sensitization; case report. Med. J. Rec. 132: 382.
77.) Rebell GC and Forster RK (1974) Fungi of keratomycosis. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 2nd edn. pp. 482. Washington, D.C.: American Society for Microbiology.
78.) Rebell GC and Forster RK (1980) Fungi of keratomycosis. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 553. Washington, D.C.: American Society for Microbiology.
79.) Reynolds JEF (Ed.) (1982) Martindale. The Extra Pharmacopoeia, 28th edn. London: Pharmaceutical Press.
80.) Robbers JE, Speedie MK and Tyler VE (1996) Pharmacognosy and Pharmacobiotechnology. Baltimore: Williams & Wilkins.
81.) Roberts SOB, Hay RJ and Mackenzie DWR (1984) A Clinician's Guide to Fungal Disease. New York: Marcel Dekker, Inc.
82.) Rogers AL (1980) Opportunistic and contaminating saprophytic fungi. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 654. Washington, D.C.: American Society for Microbiology.
83.) Romaguera C and Grimalt F (1985) Contact dermatitis to sodium fusidate. Contact Dermatitis 12: 176.
84.) Rudzki E and Rebendel P (1984) Contact sensitivity to antibiotics. Contact Dermatitis 11: 41.
85.) Russ C (1923) A skin eruption due to a mould. Lancet i: 77.
86.) Russell DW (1960) Sporidesmolide I, a metabolic product of Sporidesmium bakeri Syd. Biochimica et Biophysica Acta 45: 411.
87.) Salkin IF and Gordon MA (1977) Polymorphism of Malassezia furfur. Can. J. Microbiol. 23: 471.
88.) Sande MA and Mandell GL (1985) Antimicrobial agents. Antifungal and antiviral agents. In: Gilman AG, Goodman LS, Rall TW and Murad F (Eds) Goodman and Gilman's The Pharmacological Basis of Therapeutics, 7th edn. pp. 1219-1239. New York: Macmillan Publishing Company.
89.) Schwartz L, Tulipan L and Birmingham DJ (1957) Occupational Diseases of the Skin, 3rd edn. London: Henry Kimpton.
90.) Seaton A and Morgan WKC (1984) Hypersensitivity pneumonitis. In: Morgan WKC and Seaton A (Eds) Occupational Lung Diseases, 2nd edn. pp. 564-608. Philadelphia: WB Saunders Co.
91.) Shelley WB and Florence R (1961) Chronic urticaria due to mold hypersensitivity. A study in cross-sensitization and autoerythrocyte sensitization. Archives of Dermatology 83: 549.
92.) Silva-Hutner M and Cooper BH (1980) Yeasts of medical importance. In: Lennette EH et al. (Eds) Manual of Clinical Microbiology, 3rd edn. pp. 562. Washington, D.C.: American Society for Microbiology.
93.) Sollman TH (1957) A Manual of Pharmacology, 8th edn. Philadelphia: WB Saunders Co.
94.) Stockley IH (1985) Disulfiram-type reaction with some cephalosporins. Pharm. J. 234: 239.
95.) Storck H (1955) Ekzem durch Inhalation. Schweiz. Med. Wschr. 85: 608.
96.) Strauss JS and Kligman AM (1957) The relationship of atopic allergy and dermatitis. AMA Archives of Dermatology 75: 806.
97.) Talbot PHB (1971) Principles of Fungal Taxonomy. London: Macmillan Press Ltd.
98.) Tio TH et al. (1966) Subcutaneous phycomycosis. Archives of Dermatology 93: 550.
99.) Towey JW et al. (1932) Severe bronchial asthma apparently due to fungus spores found in maple bark. Journal of the American Medical Association 99: 453.
100.) Tschen JA, Knox JM, McGavran MH and Duncan C (1984) Chromomycosis. The association of fungal elements and wood splinters. Archives of Dermatology 120: 107-108.
101.) Tuft L (1975) Contact urticaria from cephalosporins. Archives of Dermatology 111: 1609.
102.) Tuft L et al. (1950) Atopic dermatitis I. An experimental clinical study of the role of inhalant allergens. Journal of Allergy 21: 181.
103.) Vollum DI (1977) Chromomycosis: a review. British Journal of Dermatology 96: 454.
104.) Watanabe S and Fujisawa S (1965) Eczematous hypersensitivity to molds. J. Med. Mycol. 6: 276.
105.) Watt JM and Breyer-Brandwijk MG (1962) The Medicinal and Poisonous Plants of Southern and Eastern Africa. Being an account of their medicinal and other uses, chemical composition, pharmacological effects and toxicology in man and animal, 2nd edn. Edinburgh: E & S Livingstone Ltd.
106.) White JC (1887) An Account of the Action of External Irritants upon the Skin. Boston: Cupples and Hurd.
107.) White RP (1934) The Dermatergoses or Occupational Affections of the Skin, 4th edn. London: HK Lewis.
108.) White WL and Downing MH (1947) The identity of "Metarrhizium glutinosum". Mycologia 39(5): 546-555.
109.) Wren RW (1968) Potter's New Cyclopaedia of Botanical Drugs and Preparations by Wren RC. Rustington, Sussex: Potter & Clarke, Health Science Press.
=====================================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No (42) 02/03/99 DR. JOSE LAPENTA R.
======================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela 1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo
Venezuela
1.998-2.024
Tlf: 0414-2976087 - 04127766810














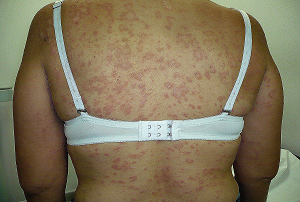




.png)