LA MINOCICLINA, LO BUENO, LO MALO Y LO FEO
Esta publicación hecha el 19 de Marzo de 1999, es APOCALÍPTICA; luego de enterarme que este antibiótico tenía y tiene la capacidad de ELIMINAR el "imbatible" y difícil de tratar MYCOBACTERIUM LEPRAE, me dedique a estudiar e investigar a fondo este medicamento, destacando sus bondades, beneficios y efectos adversos. Titule la publicación con el nombre que veis en el título: LA MINOCICLINA LO BUENO, LO MALO Y LO FEO y la lance a través de aquella "LISTA" dermatológica a los suscritos.
Para mi sorpresa por aquellas fechas se estaba realizando un Congreso Internacional de Dermatología en CHILE, y el presidente de la Sociedad de Dermatología de ese País, DR. JUAN HONEYMAN, le pareció a él y a la junta de la REVISTA (SOCHIDERM), que el artículo era demasiado interesante y lo "COLGARON" o publicaron en el reverso de la PORTADA PRINCIPAL de esa revista.
Me enteré del hecho porque una Dra. Dermatóloga de Maracay ALTANISIA RAMUNNO quien asistió a ese evento me mandó un E-MAIL, diciendome: "Te felicito Lapenta, tu publicación de la MINOCICLINA, LO BUENO LO MALO Y LO FEO, la colocaron en la revista Chilena de Dermatología..", hecho que ocurrió en Agosto de ese año.
Ningún otro médico dermatólogo de este país me comunico ese evento, solo esta Dra, a quien siempre estaré agradecido. El documento o revista nunca la pude obtener, en esa época la digitalización estaba comenzando, y al final me quedo solo esa satisfacción.
Luego me enteré que especialista a quien le publican un artículo médico en CHILE, puede ir directamente a ejercer la especialidad allí sin tanto papeleo, cosa que no se si es cierto hoy dia.
De modo que aquí les dejo la publicación tal cual original y sin cambios, la MINOCICLINA ha demostrado ser útil en muchas enfermedades incluyendo LEPRA, lo sabíais ?
Hoy 2024 este "olvidado" medicamento es DEMASIADO útil en muchas enfermedades, lo actualice en 2017 y hoy 2024 lo acomodo en la lista de este año, porque pienso que los dermatólogos del mundo deben conocer las bondades del mismo.
Aquí encuentras una actualización que hice sobre LA MINOCICLINA NUEVOS USOS PARA UN VIEJO ANTIBIÓTICO (2025,con más información y REFERENCIAS BIBLIOGRÁFICAS.
Aquí encuentras la PUBLICACIÓN INTERNACIONAL sobre LA MINOCICLINA EN LA ENFERMEDAD DE LYME (2017)
Saludos,,,
Dr. José Lapenta.
ENGLISH
This publication, made on March 19, 1999, is APOCALYPTIC; after learning that this antibiotic had and has the capacity to ELIMINATE the "unbeatable" and difficult to treat MYCOBACTERIUM LEPRAE, I dedicated myself to studying and thoroughly investigating this medicine, highlighting its advantages, benefits and adverse effects. I titled the publication with the name you see above: MINOCYCLINE THE GOOD, THE BAD AND THE UGLY, and I launched it through that dermatological "LIST" to the subscribers.
To my surprise, at that time an International Congress of Dermatology was being held in CHILE, and the president of the Dermatology Society of that country, DR. JUAN HONEYMAN, thought that the article was too interesting to him and to the board of the MAGAZINE (SOCHIDERM), and they "HANGED" it or published it on the back of the MAIN COVER of that magazine.
I found out about this, because a Dra. Dermatologist from Maracay, ALTANISIA RAMUNNO, who attended the event, sent me an E-MAIL, telling me: "I congratulate you Lapenta, your publication of MINOCYCLINE, THE GOOD, THE BAD AND THE UGLY, was placed in the Chilean Journal of Dermatology...", event that occurred in August of that year.
No other dermatologist in this country told me about this event, only this Dra., to whom I will always be grateful. I was never able to obtain the document or journal, at that time digitalization was just beginning, and in the end I was left with only that satisfaction.
Then I found out that a specialist who has a medical article published in CHILE can go directly to practice the specialty there without so much paperwork, something that I don't know if it is true today.
So here I leave you the publication as is, original and without changes, MINOCYCLINE has proven to be useful in many diseases including LEPROSY, did you know that?
Today, in 2024, this "forgotten" medicine is VERY useful in many diseases. I updated it in 2017 and today, in 2024, I am adding it to this year's list, because I think that dermatologists around the world should know its benefits.
Here you will find the update that I made on THE MINOCYCLINE NEW USES FOR AN OLD ANTIBIOTIC (2025) , with more information and BIBLIOGRAPHIC REFERENCES.
Here you can find the INTERNATIONAL PUBLICATION on MINOCYCLINE IN LYME DISEASE (2017).
Greetings...
Dr. José Lapenta R.
EDITORIAL ESPANOL:
====================
Hola amigos DERMAGICOS, el tema de hoy LA MINOCICLINA, lo bueno la malo y feo de este antibiótico. Quien iba a pensar hace unos años, que este popular medicamento iba a tener efectos beneficiosos sobre enfermedades como la lepra, esclerodermia, pénfigo, pioderma gangrenoso, penfigoide, papilomatosis reticulada y otras más. Estas 49 referencias nos ilustran bien el tema.
Y les pregunto,, realmente la minociclina se esta usando en la lepra en nuestros países, ???
Dras: Ana Rita y Maria Alejandra Jimenez (Venezuela), bienvenidas a DERMAGIC
próxima edición: Colagenosis: LA MORFEA
Saludos,,,
Dr. José Lapenta R.,,,
EDITORIAL ENGLISH:
===================
Hello DERMAGICS, friends the topic of today THE MINOCICLINA, the good thing the bad and ugly of this antibiotic. Who will have thought for some years that this popular medication will have beneficial effects on illnesses like the leprosy, scleroderma, pemphigus, gangrenous pyoderma, pemphigoid, reticulated papillomatosis and other but. This 49 references illustrate us well the topic.
And do I ask you, really the minociclina is it using in the leprosy in our countries???
Dr: Ana Rita and Maria Alejandra Jimenez (Venezuela), welcome to DERMAGIC
Next edition: THE MORPHEA (LOCALIZED SCLERODERMA)
Greetings,,,
Dr. José Lapenta R.
======================================================================
DERMAGIC/EXPRESS(45)
======================================================================
LA MINOCICLINA: LO BUENO LO MALO Y LO FEO
THE MINOCYCLINE: THE GOOD, THE BAD, AND THE UGLY.
======================================================================
======================================================================
()()()()()()()()()()()()LO BUENO () THE GOOD ()()()()()()()()()()()()()()
=========================================================================
1.) Superior antibacterial action and reduced incidence of bacterial
resistance in minocycline compared to tetracycline-treated acne patients.
2.) Tetracycline-resistant propionibacteria from acne patients are
cross-resistant to doxycycline, but sensitive to minocycline.
3.) Research letters : Minocycline in early diffuse scleroderma
4.) Minocycline in lepromatous leprosy.
5.) Efficacy of minocycline in single dose and at 100 mg twice daily for
lepromatous leprosy.
6.) Field trial on efficacy of supervised monthly dose of 600 mg rifampin,
400 mg ofloxacin and 100 mg minocycline for the treatment of leprosy; first
results.
7.) Bactericidal activity of a single-dose combination of ofloxacin plus
minocycline, with or without rifampin, against Mycobacterium leprae in mice
and in lepromatous patients.
8.) Efficacy of single dose multidrug therapy for the treatment of
single-lesion paucibacillary leprosy. Single-lesion Multicentre Trial Group.
9.) Bactericidal activity of single dose of clarithromycin plus
minocycline, with or without ofloxacin, against Mycobacterium leprae in
patients.
10.) WHO Expert Committee on Leprosy.
11.) Experimental evaluation of possible new short-term drug regimens for
treatment of multibacillary leprosy.
12.) Powerful bactericidal activities of clarithromycin and minocycline
against Mycobacterium leprae in lepromatous leprosy.
13.) Minocycline is a useful adjuvant therapy for pemphigus.
14.) Confluent and reticulated papillomatosis: response to minocycline.
15.) Minocycline treatment for confluent and reticulated papillomatosis.
16.) No detection of Borrelia burgdorferi-specific DNA in erythema migrans
lesions after minocycline treatment.
17.) Minimizing cicatricial pemphigoid orodynia with minocycline.
18.) Response of atypical bullous pyoderma gangrenosum to oral minocycline
hydrochloride and topical steroids.
19.) Blastomycosis-like pyoderma--report of a case responsive to
combination therapy utilizing minocycline and carbon dioxide laser
debridement.
20.) A sporotrichoid-like Mycobacterium kansasii infection of the skin
treated with minocycline hydrochloride.
21.) Primary lymphocutaneous nocardiosis due to Nocardia otitidiscaviarum:
the first case report from Japan.
22.) Minocycline treatment of pulmonary nocardiosis.
23.) Minocycline in rheumatoid arthritis. A 48-week, double-blind,
placebo-controlled trial. MIRA Trial Group [see comments]
24.) Minocycline prevents the decrease in bone mineral density and
trabecular bone in ovariectomized aged rats.
25.) Minocycline and cefotaxime in the treatment of experimental murine
Vibrio vulnificus infection.
26.) The role of minocycline in the treatment of intracranial 9L glioma.
27.) Evaluation of the long-term efficacy and safety of locally-applied
minocycline in adult periodontitis patients.
28.) Clinical and microbiological effects of minocycline-loaded
microcapsules in adult periodontitis.
29.) The broad-spectrum activity and efficacy of catheters coated with
minocycline and rifampin.
30.) Symptomatic hepatic cysts: treatment with single-shot injection of
minocycline hydrochloride.
======================================================================
()()()(()()()()()()()()( LO MALO () THE BAD ()()()()()()()()()()()()()()
======================================================================
31.) Minocycline induced autoimmune hepatitis and systemic lupus
erythematosus-like syndrome [see comments]
32.) Minocycline and Lupuslike Syndrome in Acne Patients
33.) Minocycline-induced lupus.
34.) Minocycline related lupus.
35.) Acute hepatitis and drug-related lupus induced by minocycline treatment.
36.) Minocycline-induced pneumonitis with bilateral hilar lymphadenopathy
and pleural effusion.
37.) Comparative safety of tetracycline, minocycline, and doxycycline.
38.) Serious adverse reactions induced by minocycline. Report of 13
patients and review of the literature.
39.) [Side effects of minocycline in the treatment of acne vulgaris]
40.) Serious dermatologic reactions in children.
41.) Serum sickness-like syndrome associated with minocycline therapy.
42.) Minocycline-induced loss of potassium from erythrocytes:
identification of a family with an augmented response.
======================================================================
()()()()()()()()()()()()()() LO FEO () THE UGLY ()()()()()()()()()()()()
======================================================================
43.) Minocycline-induced hyperpigmentation in leprosy.
44.) Psoriatic arthritis and minocycline induced autoantibodies.
45.) Minocycline hydrochloride hyperpigmentation complicating treatment of
pyoderma gangrenosum.
46.) Minocycline-induced oral pigmentation.
47.) Nodular hyperplasia, black thyroid, and chronic minocycline ingestion
in a teenager.
48.) Black bones following long-term minocycline treatment.
49.) Pigmented postacne osteoma cutis in a patient treated with
minocycline: report and review of the literature.
======================================================================
======================================================================
()()()()()()()()()()()()LO BUENO () THE GOOD ()()()()()()()()()()()()()()
======================================================================
======================================================================
1.) Superior antibacterial action and reduced incidence of bacterial
resistance in minocycline compared to tetracycline-treated acne patients.
=========================================================================
ARTICLE SOURCE: Br J Dermatol (England), Feb 1990, 122(2) p233-44
AUTHOR(S): Eady EA; Cove JH; Holland KT; Cunliffe WJ
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Twenty-five previously untreated acne patients were monitored
throughout a 6-month course of therapy with either tetracycline or
minocycline for changes in the numbers of staphylococci, propionibacteria
and yeasts of the genus Malessezia on the skin surface. Antibiotic
resistant staphylococci and propionibacteria were also counted. Minocycline
(50 mg b.d.) produced a 10-fold greater reduction in propionibacterial
numbers compared to tetracycline (500 mg b.d.) after 12 (P less than 0.02,
t-test) and 24 weeks (P less than 0.05) of therapy. As treatment
progressed, propionibacteria were replaced by yeasts, numbers of which were
significantly increased by week 12 (P less than 0.02) in
tetracycline-treated patients and by week 24 (P less than 0.01) in
minocycline-treated patients. This suggests that yeasts have no role in the
pathogenesis of acne but may compete with propionibacteria for the same
niche. Overgrowth of antibiotic resistant staphylococci prevented any
decrease in staphylococcal numbers in tetracycline-treated patients, but
minocycline produced a significant and sustained reduction in
staphylococcal numbers after 1 week of therapy (P less than 0.001). An
increase in the number of multiply resistant (greater than or equal to 3
resistances) staphylococci occurred in 67% of tetracycline-treated and 33%
of minocycline-treated patients by the end of the treatment period. There
was no evidence of propionibacterial resistance in either treatment group.
This study shows that minocycline has much greater antibacterial activity
in vivo against both staphylococci and propionibacteria and produces less
staphylococcal antibiotic resistance than tetracycline.
=========================================================================
2.) Tetracycline-resistant propionibacteria from acne patients are
cross-resistant to doxycycline, but sensitive to minocycline.
=========================================================================
ARTICLE SOURCE: Br J Dermatol (England), May 1993, 128(5) p556-60
AUTHOR(S): Eady EA; Jones CE; Gardner KJ; Taylor JP; Cove JH; Cunliffe WJ
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Antibiotic-resistant propionibacteria are being isolated with
increasing frequency from antibiotic-treated acne patients. Minimum
inhibitory concentrations (MICs) of three tetracyclines, extensively used
in acne therapy, were determined for 46 resistant and 19 sensitive
propionibacteria isolates. Sensitive strains were inhibited by or = 1
microgram/ml of all three tetracyclines. For every resistant strain tested,
the MIC of tetracycline exceeded that of doxycycline which, in turn,
exceeded that of minocycline. The mean MIC for resistant strains was 20.61
+/- 4.56 micrograms/ml of tetracycline, 9.70 +/- 2.03 micrograms/ml of
doxycycline and 1.95 +/- 0.35 micrograms/ml of minocycline. In order to
determine whether these strains could be inhibited by concentrations of
minocycline achievable in vivo, serum levels of minocycline were determined
in acne patients receiving either the recommended dose of 50 mg b.d. (20
males, 14 females), or twice this dose (21 males, 12 females). Serum levels
were significantly higher (P 0.001, Student's t-test) in patients receiving
100 mg b.d. Males on 50 mg b.d. had significantly lower serum levels than
females on the same dose (P 0.05. Student's t-test). For all patients, the
mean serum level on high-dose minocycline was 2.46 +/- 0.45 micrograms/ml,
compared with 1.38 +/- 0.30 micrograms/ml on the smaller dose. These
results indicate that tetracycline-resistant propionibacteria should be
considered clinically minocycline sensitive, if patients who harbour such
strains are prescribed 100 mg b.d. The recommended dose of minocycline for
treating acne, especially in male patients, should be re-assessed.
=========================================================================
3.) Research letters : Minocycline in early diffuse scleroderma
=========================================================================
The Lancet 1998;352(9142):1755-6
Minocycline may effectively treatearly diffuse scleroderma. 11 patients in
the early stages of skin disease treated in open-label fashion for up to one
year with minocycline. Six patients completed the study, of whom four had
complete resolution of disease.
=========================================================================
4.) Minocycline in lepromatous leprosy.
=========================================================================
Author
Fajardo TT Jr; Villahermosa LG; dela Cruz EC; Abalos RM; Franzblau SG;
Walsh GP
Address
Clinical Research Branch, Leonard Wood Memorial Center, Cebu City, The
Philippines.
Source
Int J Lepr Other Mycobact Dis, 63(1):8-17 1995 Mar
Abstract
Twelve patients were treated with three dose levels of minocycline for 30
days, primarily to detect the dose-related effects on Mycobacterium leprae
viability, followed by another 5 months of daily minocycline for overall
efficacy and persistence of clinical and antibacterial effects.
Subsequently, the patients were given standard WHO/MDT chemotherapy for
multibacillary leprosy. Clinical improvement was recognizable during the
first month, occurring much earlier among those on minocycline 200 mg daily
than those who received minocycline 100 mg daily. A similar change also was
observed in one patient 11 days after three daily doses of 100 mg of
minocycline. At the end of 6 months, all patients were clinically improved
with a slight reduction in the average bacterial index (BI) and logarithmic
index of bacilli in biopsy (LIB). The effects of minocycline on viability
by mouse foot pad inoculation and palmitic acid oxidation assays were noted
beginning at 10 to 14 days of daily dosing and becoming more definite after
30 days of treatment. Both tests correlated fairly well. Doses of 200 mg
daily did not appear to be more efficient than minocycline 100 daily.
Phenolic glycolipid-I (PGL-I) antigen determinations done on some patients
during the first month remained positive and did not correlate with changes
in viability results. At the end of 6 months, after 5 months of 100 mg of
minocycline monotherapy, no viable organisms could be demonstrated by mouse
foot pad inoculation and palmitic acid oxidation assays; assays for PGL-I
antigen were all negative.(ABSTRACT TRUNCATED AT 250 WORDS)
=========================================================================
5.) Efficacy of minocycline in single dose and at 100 mg twice daily for
lepromatous leprosy.
=========================================================================
Int J Lepr Other Mycobact Dis (United States), Dec 1994, 62(4) p568-73
AUTHOR(S): Gelber RH; Murray LP; Siu P; Tsang M; Rea TH
AUTHOR'S ADDRESS: San Francisco Regional Hansen's Disease Program, CA 94115.
PUBLICATION TYPE: CLINICAL TRIAL; JOURNAL ARTICLE
ABSTRACT: A clinical trial of minocycline in a total of 10 patients with
previously untreated lepromatous leprosy was conducted in order to evaluate
the efficacy of a single, initial, 200-mg dose and 100 mg twice daily of
minocycline for a total duration of up to 3 months. Patients improved
remarkably quickly. Although single-dose therapy did not result in a
significant killing of Mycobacterium leprae, viable M. leprae were cleared
from the dermis regularly by 3 months of twice-daily therapy, a rate
similar to that achieved by minocycline 100 mg once daily. Because more
side effects were noted herein than previously with 100 mg daily, we
recommend that minocycline, when applied, be administered at 100 mg daily
to leprosy patients.
=========================================================================
6.) Field trial on efficacy of supervised monthly dose of 600 mg rifampin,
400 mg ofloxacin and 100 mg minocycline for the treatment of leprosy; first
results.
=========================================================================
Author
Mane I; Cartel JL; Grosset JH
Address
Institut de Leprologie Appliquee, Dakar CD Annexe, Senegal.
Source
Int J Lepr Other Mycobact Dis, 65(2):224-9 1997 Jun
Abstract
In 1995, a field trial was implemented in Senegal in order to evaluate the
efficacy of a regimen based on the monthly supervised intake of rifampin
600 mg, ofloxacin 400 mg and minocycline 100 mg to treat leprosy. During
the first year of the trial, 220 patients with active leprosy (newly
detected or relapsing after dapsone monotherapy) were recruited: 102
paucibacillary (PB) (60 males and 42 females) and 118 multibacillary (MB)
(71 males and 47 females). All of them accepted the new treatment (none
requested to be preferably put under standard WHO/MDT), no clinical sign
which could be considered as a toxic effect of the drug was noted, and none
of the patients refused to continue treatment because of any clinical
trouble. The compliance was excellent: the 113 patients (PB and MB)
detected during the first 6 months of the trial have taken six monthly
doses in 6 months, as planned. The rate of clearance and the progressive
decrease of cutaneous lesions was satisfactory. Although it is too soon to
give comprehensive results, it should be noted that no treatment failure
was observed in the 56 PB patients who have completed treatment and have
been followed up for 6 months. The long-term efficacy of the new regimen is
to be evaluated on the rate of relapse during the years following the
cessation of treatment. If that relapse rate is acceptable (similar to that
observed in patients after treatment with current standard WHO/ MDT), the
new regimen could be a solution to treat, for instance, patients very
irregular and/or living in remote or inaccessible areas since no selection
of rifampin-resistant Mycobacterium leprae should be possible (a monthly
dose of ofloxacin and minocycline being as effective as a dose of dapsone
and clofazimine taken daily for 1 month). Nevertheless, until longer term
results of this and other trials become available, there is no
justification for any change in the treatment strategy, and all leprosy
patients should be put under standard WHO/MDT.
=========================================================================
7.) Bactericidal activity of a single-dose combination of ofloxacin plus
minocycline, with or without rifampin, against Mycobacterium leprae in mice
and in lepromatous patients.
=========================================================================
Author
Ji B; Sow S; Perani E; Lienhardt C; Diderot V; Grosset J
Address
Facult´e de M´edecine Piti´e-Salp^etri`ere, Paris, France.
bacterio@biomath.jussieu.fr
Source
Antimicrob Agents Chemother, 42(5):1115-20 1998 May
Abstract
To develop a fully supervisable, monthly administered regimen for treatment
of leprosy, the bactericidal effect of a single-dose combination of
ofloxacin (OFLO) and minocycline (MINO), with or without rifampin (RMP),
against Mycobacterium leprae was studied in the mouse footpad system and in
previously untreated lepromatous leprosy patients. Bactericidal activity
was measured by the proportional bactericidal method. In mouse experiments,
the activity of a single dose of the combination OFLO-MINO was dosage
related; the higher dosage of the combination displayed bactericidal
activity which was significantly inferior to that of a single dose of RMP,
whereas the lower dosage did not exhibit a bactericidal effect. In the
clinical trial, 20 patients with previously untreated lepromatous leprosy
were treated with a single dose consisting of either 600 mg of RMP plus 400
mg of OFLO and 100 mg of MINO or 400 mg of OFLO plus 100 mg of MINO. The
OFLO-MINO combination exhibited definite bactericidal activity in 7 of 10
patients but was less bactericidal than the RMP-OFLO-MINO combination. Both
combinations were well tolerated. Because of these promising results, a
test of the efficacy of multiple doses of ROM in a larger clinical trial
appears justified.
=========================================================================
8.) Efficacy of single dose multidrug therapy for the treatment of
single-lesion paucibacillary leprosy. Single-lesion Multicentre Trial Group.
=========================================================================
Source
Indian J Lepr, 69(2):121-9 1997 Apr-Jun
Abstract
A multicentre double-blind controlled clinical trial was carried out to
compare the efficacy of a combination of rifampicin 600 mg plus ofloxacin
400 mg plus minocycline 100 mg (ROM) administered as single dose with that
of the standard six-month WHO/MDT/PB regimen. The subjects included 1483
cases with one skin lesion who were previously untreated, were
smear-negative, and had no evidence of peripheral nerve trunk involvement,
and they were randomly divided into study and control groups. The total
duration of the study from the day of intake was 18 months, and 1381
patients completed study. Only 12 patients were categorized as treatment
failure and no difference was observed between the two regimens. Occurrence
of mild side-effects and leprosy reactions were minimal (less than 1%) in
both groups. This study showed that ROM is almost as effective as the
standard WHO/MDT/PB in the treatment of single lesion PB leprosy.
=========================================================================
9.) Bactericidal activity of single dose of clarithromycin plus
minocycline, with or without ofloxacin, against Mycobacterium leprae in
patients.
=========================================================================
Author
Ji B; Jamet P; Perani EG; Sow S; Lienhardt C; Petinon C; Grosset JH
Address
Facult´e de M´edecine Piti´e-Salp^etri`ere, Paris, France.
Source
Antimicrob Agents Chemother, 40(9):2137-41 1996 Sep
Abstract
Fifty patients with newly diagnosed lepromatous leprosy were allocated
randomly to one of five groups and treated with either a month-long
standard regimen of multidrug therapy (MDT) for multibacillary leprosy, a
single dose of 600 mg of rifampin, a month-long regimen with the dapsone
(DDS) and clofazimine (CLO) components of the standard MDT, or a single
dose of 2,000 mg of clarithromycin (CLARI) plus 200 mg of minocycline
(MINO), with or without the addition of 800 mg of ofloxacin (OFLO). At the
end of 1 month, clinical improvement accompanied by significant decreases
of morphological indexes in skin smears was observed in about half of the
patients of each group. A significant bactericidal effect was demonstrated
in the great majority of patients in all five groups by inoculating the
footpads of mice with organisms recovered from biopsy samples obtained
before and after treatment. Rifampin proved to be a bactericidal drug
against Mycobacterium leprae more potent than any combination of the other
drugs. A single dose of CLARI-MINO, with or without OFLO, displayed a
degree of bactericidal activity similar to that of a regimen daily of doses
of DDS-CLO for 1 month, suggesting that it may be possible to replace the
DDS and CLO components of the MDT with a monthly dose of CLARI-MINO, with
or without OFLO. However, gastrointestinal adverse events were quite
frequent among patients treated with CLARI-MINO, with or without OFLO, and
may be attributed to the higher dosage of CLARI or MINO or to the
combination of CLARI-MINO plus OFLO. In future trials, therefore, we
propose to reduce the dosages of the drugs to 1,000 mg of CLARI, 100 mg of
MINO, and 400 mg of OFLO.
=========================================================================
10.) WHO Expert Committee on Leprosy.
=========================================================================
Source: World Health Organ Tech Rep Ser, 874():1-43 1998
Abstract
Considerable progress has been made in the fight against leprosy during the
past 10-15 years, following the introduction of multidrug therapy (MDT)
regimens and the establishment of the goal of eliminating leprosy as a
public health problem by the year 2000. Current estimates indicate that
there are about 1.15 million cases of leprosy in the world, compared with
10-12 million cases in the mid-1980s. This report presents the conclusions
of a WHO Expert Committee convened to review the global leprosy situation
and the technology available for eliminating the disease, to identify the
remaining obstacles to reaching the goal of eliminating leprosy as a public
health problem, and to make appropriate recommendations for the future on
technical and operational matters. The current status of leprosy
elimination is discussed, and the various antileprosy drugs are reviewed,
including the most recently available drugs. On the basis of field trials
and clinical studies, the Committee concludes that a single dose of a
combination of rifampicin, ofloxacin and minocycline is an acceptable and
cost-effective alternative regimen for the treatment of single-lesion
paucibacillary leprosy, and that the duration of the current MDT regimen
for multibacillary leprosy could possibly be shortened to 12 months. The
Committee points out the need for improved management of reactions and
neuritis and prevention of leprosy-related disabilities and impairments,
and recommends that antileprosy activities should become an integral part
of general health services and should involve communities to the fullest
extent possible.
=========================================================================
11.) Experimental evaluation of possible new short-term drug regimens for
treatment of multibacillary leprosy.
=========================================================================
Author
Banerjee DK; McDermott-Lancaster RD; McKenzie S
Address
Department of Medical Microbiology, St George's Hospital Medical School,
London, United Kingdom. banerjee@sghms.ac.uk.
Source
Antimicrob Agents Chemother, 41(2):326-30 1997 Feb
Abstract
Groups of nude mice, with both hind footpads infected with 10(8)
Mycobacterium leprae organisms, were treated with 4-week courses of
different drug combinations. The effect treatment on each group was
evaluated by subinoculating footpad homogenates from the treated mice into
groups of normal and nude mice for subsequent regrowth, assessed 1 year
later. A combination of rifampin (RMP) with clarithromycin (CLARI),
minocycline (MINO), and ofloxacin (OFLO) resulted in the complete killing
of M. leprae after 3 weeks of treatment. A combination of sparfloxacin
(SPAR) and RMP also resulted in a similar bactericidal effect after 3 weeks
of treatment. Other drug combinations showed variable effects. Very little
or no effect was observed with any regimen if the treatment was given for
less than 2 weeks. World Health Organization (WHO) multidrug therapy (MDT)
given for 8 weeks was as effective as the two combinations described above.
The results suggest that multidrug combinations consisting of RMP-OFLO (or
SPAR)-CLARI (and/or MINO) are as effective as the WHO MDT for the treatment
of experimental leprosy. Moreover, they imply that these combinations,
which were found to be active in a 4-week experimental treatment protocol,
could be administered as treatment to patients for a period of time shorter
than the present 2-year regimen without a loss of effectiveness.
=========================================================================
12.) Powerful bactericidal activities of clarithromycin and minocycline
against Mycobacterium leprae in lepromatous leprosy.
=========================================================================
ARTICLE SOURCE: J Infect Dis (United States), Jul 1993, 168(1) p188-90
AUTHOR(S): Ji B; Jamet P; Perani EG; Bobin P; Grosset JH
AUTHOR'S ADDRESS: Faculte de Medecine Pitie-Salpetriere, Paris, France.
PUBLICATION TYPE: CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED
TRIAL
ABSTRACT: Thirty-six patients with newly diagnosed lepromatous leprosy
were allocated randomly to three groups and treated for 56 days with
minocycline (100 mg daily), clarithromycin (500 mg daily), or
clarithromycin (500 mg) plus minocycline (100 mg daily). All groups had
rapid and remarkable clinical improvement and significant decline of the
bacterial and morphologic indices in skin smears during treatment. More
than 99% and 99.9% of the viable Mycobacterium leprae had been killed by 28
and 56 days of treatment, respectively, as measured by inoculation of
organisms recovered from skin samples, taken before and during treatment,
into the footpads of immunocompetent and nude mice. Clinical improvement
and bactericidal activity did not differ significantly among the three
groups. Adverse reactions were rare and mild, and no laboratory abnormality
was detected during the trial. Both clarithromycin and minocycline
displayed powerful bactericidal activities against M. leprae in leprosy
patients and may be considered important components of new multidrug
regimens for the treatment of multibacillary leprosy.
=========================================================================
13.) Minocycline is a useful adjuvant therapy for pemphigus.
=========================================================================
Author
Gaspar ZS; Walkden V; Wojnarowska F
Address
Department of Dermatology, Churchill, Oxford Radcliffe Hospital,
Headington, UK.
Source
Australas J Dermatol, 37(2):93-5 1996 May
Abstract
Pemphigus is an autoimmune blistering disease with high mortality if
untreated. The cases of 10 patients who had minocycline 100 mg daily added
as adjuvant therapy are reported. Prior to the use of minocycline, all
patients had active disease, nine were on prednisolone (10-40 mg) and five
were on azathioprine (100-200 mg). The response was assessed on clinical
improvement and reduction of immunosuppressive (IS) drugs. It was graded
into four categories: major, minor, equivocal and no significant response.
A major response was seen in four patients, minor in two, equivocal in one
and no improvement in three patients. The prednisolone dose in the six
responders was reduced to 0-6 mg (0 mg in three patients), with an average
decrease of 21 mg. The average time to respond was 8 months. Of the six
responders, three were on azathioprine, which was ceased in two patients
and reduced by two-thirds in the other patient. No patient ceased
minocycline because of side effects. In conclusion, minocycline 100 mg
daily is a simple, safe and well tolerated treatment that should be tried
in patients with pemphigus to reduce disease activity and/or the dose of
potent IS agents.
=========================================================================
14.) Confluent and reticulated papillomatosis: response to minocycline.
=========================================================================
Author
Montemarano AD; Hengge M; Sau P; Welch M
Address
Department of Dermatology, Walter Reed Army Medical Center, Washington, DC
20307, USA.
Source
J Am Acad Dermatol, 34(2 Pt 1):253-6 1996 Feb
Abstract
BACKGROUND: Confluent and reticulated papillomatosis (CRP) of Gougerot and
Carteaud is an uncommon disorder of unknown cause for which a variety of
treatments have been proposed. OBJECTIVE: We attempted to evaluate the
effectiveness of oral minocycline. METHODS: Nine patients with CRP were
treated with oral minocycline, 50 mg twice a day, for 6 weeks. The average
follow-up period was 11 months. Recurrence rate, side effects, and
effectiveness of therapy were assessed. RESULTS: All patients except two
had a 90% to 100% response to therapy. Recurrences were noted in three
patients, all of whom responded to re-treatment with minocycline. None of
the nine patients had an adverse reaction. CONCLUSION: Minocycline, 50 mg
twice a day, is safe and effective for CRP.
=========================================================================
15.) Minocycline treatment for confluent and reticulated papillomatosis.
=========================================================================
Author
Chang SN; Kim SC; Lee SH; Lee WS
Address
Department of Dermatology, Yonsei University College of Medicine, Seoul,
Korea.
Source
Cutis, 57(6):454-7 1996 Jun
Abstract
We report six cases of confluent and reticulated papillomatosis in which
the skin lesions cleared almost completely after treatment with
minocycline. Patients with confluent and reticulated papillomatosis often
do not respond well to a variety of therapeutic agents. The response of
confluent and reticulated papillomatosis to minocycline was first described
in 1965. Although the pharmacologic mechanisms of minocycline in confluent
and reticulated papillomatosis are unknown, we suggest that it should be
considered as a first-choice therapeutic agent because of its marked
effectiveness.
=========================================================================
16.) No detection of Borrelia burgdorferi-specific DNA in erythema migrans
lesions after minocycline treatment.
=========================================================================
ARTICLE SOURCE: Arch Dermatol (United States), Jun 1995, 131(6) p678-82
AUTHOR(S): Muellegger RR; Zoechling N; Soyer HP; Hoedl S; Wienecke R;
Volkenandt M; Kerl H
AUTHOR'S ADDRESS: Department of Dermatology, Karl-Franzens University,
Graz, Austria.
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: BACKGROUND AND DESIGN: Early treatment of erythema migrans is
important to prevent late complications. Minocycline possesses several
attributes, making it potentially useful in the treatment of borrelial
infections. In our study, minocycline was administered to 14 patients with
erythema migrans. Punch biopsy specimens were obtained from the (affected)
skin of all patients before and after therapy. The formalin-fixed,
paraffin-embedded specimens were analyzed by polymerase chain reaction for
the presence of Borrelia burgdorferi-specific DNA. RESULTS: Polymerase
chain reaction assay succeeded in amplifying B burgdorferi-specific DNA
from the first biopsy specimen, obtained from the border of erythema
migrans before initiating treatment, in eight (57%) of 14 patients. At the
end of minocycline therapy, however, polymerase chain reaction analysis
disclosed no B burgdorferi-specific DNA in any of the 14 patients. The good
clinical response of our patients with erythema migrans substantiates our
molecular findings. CONCLUSIONS: The presented polymerase chain reaction
data, together with the clinical outcome, indicate that minocycline may be
useful for treatment of early Lyme borreliosis.
=========================================================================
17.) Minimizing cicatricial pemphigoid orodynia with minocycline.
=========================================================================
ARTICLE SOURCE: Br J Dermatol (England), May 1995, 132(5) p784-9
AUTHOR(S): Poskitt L; Wojnarowska F
AUTHOR'S ADDRESS: Dermatology Department, Amersham General Hospital,
Bucks, U.K.
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Cicatricial pemphigoid is a rare autoimmune blistering disease
of the elderly. It predominantly affects the mucosae, causing pain and
scarring. the target antigen is within the lamina lucida of the basement
membrane zone. Potential complications of systemic steroid and other
immunosuppressive therapy have prompted trials of other means of treatment.
We describe a series of seven patients treated with minocycline, six of
whom derived sustained alleviation of orodynia. Four patients developed
hyperpigmentation, and two complained of gastrointestinal discomfort which
necessitated cessation of minocycline. Complete steroid withdrawal was
achieved in two cases. Neither the disease progression nor the response to
treatment was influenced by the immunoglobulin isotype or titre. The role
of minocycline as a useful adjunct to therapy is discussed.
=========================================================================
18.) Response of atypical bullous pyoderma gangrenosum to oral minocycline
hydrochloride and topical steroids.
=========================================================================
ARTICLE SOURCE: Acta Derm Venereol (Sweden), 1990, 70(6) p538-9
AUTHOR(S): Reynolds NJ; Peachey RD
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: An 80-year-old Caucasian female with rheumatoid arthritis and
recurrent atypical bullous pyoderma gangrenosum is described. There was no
evidence of any underlying myeloproliferative disorder. Rapid healing
occurred in response to oral minocycline hydrochloride and topical
clobetasol propionate.
=========================================================================
19.) Blastomycosis-like pyoderma--report of a case responsive to
combination therapy utilizing minocycline and carbon dioxide laser
debridement.
=========================================================================
ARTICLE SOURCE: J Dermatol Surg Oncol (United States), Oct 1986, 12(10)
p1041-4
AUTHOR(S): Sawchuk WS; Heald PW
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Blastomycosis-like pyoderma is an uncommon reaction pattern to a
superficial bacterial infection in persons with a variety of predisposing
conditions such as chronic ethanol use and poor nutrition. We are reporting
a case that initially responded poorly to previously described treatment
regimens but responded well to combination treatment with carbon dioxide
laser debridement and long-term minocycline.
=========================================================================
20.) A sporotrichoid-like Mycobacterium kansasii infection of the skin
treated with minocycline hydrochloride.
=========================================================================
ARTICLE SOURCE: Br J Dermatol (England), Jul 1979, 101(1) p75-9
AUTHOR(S): Dore N; Collins JP; Mankiewicz E
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: A sporotrichoid-like Mycobacterium kansasii infection of the
skin is reported. This is the fifth reported case in the English literature
of dermatological manifestations of a M. kansasii infection and the first
reported case of a response to minocycline hydrochloride therapy.
=========================================================================
21.) Primary lymphocutaneous nocardiosis due to Nocardia otitidiscaviarum:
the first case report from Japan.
=========================================================================
ARTICLE SOURCE: J Dermatol (Japan), May 1995, 22(5) p344-7
AUTHOR(S): Suzuki Y; Toyama K; Utsugi K; Yazawa K; Mikami Y; Fujita M;
Shinkai H
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: We report a case of lymphocutaneous syndrome caused by Nocardia
otitidiscaviarum (formerly known as N. caviae) in a 78-year-old woman who
underwent long-term therapy with prednisolone for bronchial asthma.
Histological examination showed granulomatous reaction with multiple
polymorphonuclear leukocytes and revealed a Gram positive filament in the
dermis. Gram-positive, slightly acid-fast branched filaments were also
found in the smear of the purulent material. The cell wall constituents of
the isolate were meso-diaminopimelic acid, arabinose, and galactose; the
mycolic acid pattern of the isolate was Nocardia type. The organism
decomposed xanthine and hypoxanthine, but not tyrosine or casein, which
distinguished it from N. asteroides and N. brasiliensis. The skin lesions
responded to minocycline and later to a combination of doxycycline and
ofloxacin. This primary lymphocutaneous nocardiosis due to N.
otitidiseaviarum is the first in Japan.
=========================================================================
22.) Minocycline treatment of pulmonary nocardiosis.
=========================================================================
ARTICLE SOURCE: JAMA (United States), Aug 19 1983, 250(7) p930-2
AUTHOR(S): Petersen EA; Nash ML; Mammana RB; Copeland JG
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Minocycline hydrochloride was used to treat pulmonary infections
with Nocardia asteroides in five cardiac allograft recipients. In three
patients, minocycline was successfully used as the only antinocardial
agent. Two other patients were found to have leukopenia after initial
therapy with sulfisoxazole. These two patients were subsequently treated
with minocycline. The clinical success with minocycline in these highly
immunosuppressed patients suggests that minocycline is an effective
antinocardial agent. These data did not allow any conclusion regarding
which drug, minocycline or sulfisoxazole, is superior in the treatment of
this disease.
=========================================================================
23.) Minocycline in rheumatoid arthritis. A 48-week, double-blind,
placebo-controlled trial. MIRA Trial Group [see comments]
=========================================================================
Author
Tilley BC; Alarc´on GS; Heyse SP; Trentham DE; Neuner R; Kaplan DA; Clegg
DO; Leisen JC; Buckley L; Cooper SM; et al
Address
Henry Ford Health Sciences Center, Detroit, Michigan.
Source
Ann Intern Med, 122(2):81-9 1995 Jan 15
Abstract
OBJECTIVE: To assess the safety and efficacy of minocycline in the
treatment of rheumatoid arthritis. DESIGN: A double-blind, randomized,
multicenter, 48-week trial of oral minocycline (200 mg/d) or placebo.
SETTING: 6 clinical centers in the United States. PATIENTS: 219 adults with
active rheumatoid arthritis who had previous limited treatment with
disease-modifying drugs. MEASUREMENTS: As the primary outcomes, 60
diarthrodial joints were examined for tenderness, and 58 joints were
examined for swelling (hips excluded). Grip strength, evaluator's global
assessment, morning stiffness, Modified Health Assessment Questionnaire,
patient's global assessment, hematocrit, erythrocyte sedimentation rate,
platelet count, and IgM rheumatoid factor levels were also assessed;
radiographs of both hands and wrists were taken. RESULTS: 109 and 110
patients were randomly assigned to receive minocycline and placebo,
respectively. At entry, demographic, clinical, and laboratory measurements
were similar in both groups. Most patients had mild to moderate disease
activity and some evidence of destructive disease. At the week 48 visit,
79% of the minocycline group and 78% of the placebo group continued to
receive the study medication. At 48 weeks, more patients in the minocycline
group than in the placebo group showed improvement in joint swelling (54%
and 39%) and joint tenderness (56% and 41%) (P < 0.023 for both
comparisons). The minocycline group also showed greater improvement in
hematocrit, erythrocyte sedimentation rate, platelet count, and IgM
rheumatoid factor levels (all P values < 0.001), and more patients
receiving minocycline had laboratory values within normal ranges at 48
weeks. For the remaining outcomes, P values ranged from 0.04 to 0.76, all
greater than the critical value of 0.005 (Bonferroni adjustment for
multiple comparisons). The frequency of reported side effects was similar
in both groups, and no serious toxicity occurred. CONCLUSIONS: Minocycline
was safe and effective for patients with mild to moderate rheumatoid
arthritis. Its mechanisms of action remain to be determined.
=========================================================================
24.) Minocycline prevents the decrease in bone mineral density and
trabecular bone in ovariectomized aged rats.
=========================================================================
Author
Williams S; Wakisaka A; Zeng QQ; Barnes J; Martin G; Wechter WJ; Liang CT
Address
Gerontology Research Center, National Institute of Aging, National
Institutes of Health, Baltimore, MD 21224, USA.
Source
Bone, 19(6):637-44 1996 Dec
Abstract
In the current study, we examined the effects of minocycline, on the
osteopenia of ovariectomized aged rats. Old female rats were randomly
divided into five groups: sham, ovariectomized control and ovariectomized
treated with minocycline, 17beta-estradiol, or both agents. Bone samples
were collected 8 wk after the treatment. Ovariectomy reduced bone mineral
density of the whole femur and at the condylar, distal metaphyseal and
head-neck-trochanter regions 10%-19% and the loss of bone density was
prevented by treatment with minocycline or 17beta-estradiol.
Histomorphometric analysis of distal femur showed ovariectomy reduced the
trabecular bone area, the trabecular bone number, trabecular bone thickness
and increased the trabecular bone separation. The microanatomic structure
of trabecular bone also showed that the number of nodes, node to node,
cortical to node, node to free end was reduced by ovariectomy. Treatment
with minocycline attenuated the effect of ovariectomy on trabecular bone in
aged animals. In contrast, cortical bone was not affected by ovariectomy or
minocycline treatment. The effect of minocycline on bone turnover was also
examined. Minocycline increased osteoid surface, mineralizing surface,
mineral apposition rate, bone formation rate and reduced eroded surface. We
have therefore concluded that the modest increase in bone mineral density
and the improvement in the trabecular bone status noted in minocycline
treated ovariectomized aged rats is likely due to an increase in bone
formation coupled with a decrease in bone resorption.
=========================================================================
25.) Minocycline and cefotaxime in the treatment of experimental murine
Vibrio vulnificus infection.
=========================================================================
Author
Chuang YC; Ko WC; Wang ST; Liu JW; Kuo CF; Wu JJ; Huang KY
Address
Department of Internal Medicine, National Cheng Kung University Hospital,
Tainan, Taiwan.
Source
Antimicrob Agents Chemother, 42(6):1319-22 1998 Jun
Abstract
We conducted an in vivo study with the mouse model of Vibrio vulnificus
infection to evaluate the efficacies of therapy with minocycline or
cefotaxime alone and in combination. V. vulnificus was introduced
subcutaneously into the area over the right thigh. The inoculum size ranged
from 1.0 x 10(3) to 1.2 x 10(8) CFU from experiment to experiment but was
constant for all animals in the same experiment. Antibiotics were given
intraperitoneally 2 h after the bacteria were inoculated. In experiments 1
to 4, the standard dose for humans was used to treat the infection, while
in experiment 5, five times the standard dose for humans was used to treat
the infection. In experiment 1, with a small inoculum of 5 x 10(3) CFU, all
mice in the saline-treated control group and the cefotaxime-, minocycline-,
and combined antibiotic-treated groups survived. In experiment 2, with a
moderate inoculum of 1.2 x 10(5) CFU, all the mice in the three
antibiotic-treated groups survived, while only two of nine mice in the
control group survived. In experiment 3, with a large inoculum of 8.0 x
10(7) CFU, six of nine mice in the combined antibiotic-treated group
survived, while only one of nine mice in the cefotaxime-treated group and
none of the mice in the control and minocycline-treated groups survived. In
experiment 4, with a large inoculum of 1.2 x 10(8) CFU, 8 of 20 mice in the
combined antibiotic-treated group survived, while none of the 20 mice in
the control group, the group treated with cefotaxime alone, and the group
treated with minocycline alone survived. In experiment 5, in which mice
were infected with a large inoculum of 6.6 x 10(7) CFU and treated with
five times the standard human dose of antibiotics, 10 of 12 mice in the
combined antibiotic-treated group survived, while only 4 of 12 mice in the
minocycline-treated group, 1 of 12 mice in the cefotaxime-treated group,
and none of the mice in the control group survived. In experiments 3 to 5,
the difference in the survival rates between the combined
antibiotic-treated and minocycline-treated groups was statistically
significant (P < 0.05). These results indicate that combination therapy
with cefotaxime and minocycline is distinctly more advantageous than
therapy with the single antibiotic regimen for the treatment of severe
experimental V. vulnificus infections.
=========================================================================
26.) The role of minocycline in the treatment of intracranial 9L glioma.
=========================================================================
Author
Weingart JD; Sipos EP; Brem H
Address
Department of Neurological Surgery, Johns Hopkins University School of
Medicine, Baltimore, Maryland.
Source
J Neurosurg, 82(4):635-40 1995 Apr
Abstract
This study was designed to explore the question of whether minocycline, a
semisynthetic tetracycline shown to inhibit tumor-induced angiogenesis,
could control the growth of the rat intracranial 9L gliosarcoma.
Minocycline was tested alone and in combination with
1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in vivo. Treatment was started
at the time of intracranial implantation of 9L gliosarcoma into male
Fischer 344 rats, 5 days later, or after tumor resection. Minocycline was
delivered locally with a controlled-release polymer or systemically by
intraperitoneal injection. Systemic minocycline did not extend survival
time. Local treatment with minocycline by a controlled-release polymer
implanted at the time of tumor implantation extended median survival time
by 530% (p < 0.001) compared to treatment with empty polymer. When
treatment was begun 5 days after tumor implantation, minocycline delivered
locally or systemically had no effect on survival. However, after tumor
resection, treatment with locally delivered minocycline resulted in a 43%
increase in median survival time (p < 0.002) compared to treatment with
empty polymer. Treatment with a combination of minocycline delivered
locally in a controlled-release polymer and systemic BCNU 5 days after
tumor implantation resulted in a 93% extension of median survival time
compared to BCNU alone (p < 0.002). In contrast, treatment with a
combination of systemic minocycline and BCNU did not increase survival time
compared to systemic BCNU alone. These results demonstrate that minocycline
affects tumor growth when delivered locally and suggest that minocycline
may be a clinically effective modulator of intracranial tumor growth when
used in combination with a chemotherapeutic agent and surgical resection.
=========================================================================
27.) Evaluation of the long-term efficacy and safety of locally-applied
minocycline in adult periodontitis patients.
=========================================================================
Author
Timmerman MF; van der Weijden GA; van Steenbergen TJ; Mantel MS; de Graaff
J; van der Velden U
Address
Department of Periodontology, Academic Centre for Dentistry, Amsterdam, The
Netherlands.
Source
J Clin Periodontol, 23(8):707-16 1996 Aug
Abstract
The objectives of the present study were to establish in a long-term
investigation the safety as well as the clinical and microbiological
efficacy of scaling and rootplaning combined with local application of 2%
minocycline hydrochloride-gel versus placebo-gel in patients with moderate
to severe chronic adult periodontitis. This was an 18 months, randomized,
double-blind, parallel, comparative study, in which 20 healthy patients
with moderate to severe chronic periodontitis participated. At baseline,
all patients received professional oral hygiene-instruction and supra- and
subgingival scaling and root planing. The minocycline-gel was applied
subgingivally baseline, 2 weeks, 1, 3, 6, 9 and 12 months. Microbiological
evaluation was carried out using DMDx to identify the following bacteria:
Porphyromonas gingivalis, Prevotella intermedia, Actinobacillus
actinomycetemcomitans, Campylobacter rectus, Fusobacterium nucleatum and
Treponema denticola. In addition standard microbiological techniques were
used for the detection of P. gingivalis, P. intermedia, P. micros, A.
actinomycetemcomitans, C. rectus, F. nucleatum, C. albicans and
Enterobacteriaceae. Results showed a statistically significant improvement
for all clinical parameters irrespective of the treatment modality. No
differences were observed between test and control with regard to probing
depth and attachment level. The DMDx data showed a significant reduction in
both the numbers and the prevalence over the 15 months period, but no
significant difference between groups. Culture data showed that at baseline
two-third were positive for P. gingivalis and P. intermedia. Analysis over
the 18 month period showed no significant difference between the two
treatment modalities. C. albicans and Enterobacteriaceae were detected only
in small proportions at each time interval in a limited number of patients.
No adverse reactions were observed during the trial period. The present
patient group responded favourably to scaling and rootplaning, but did not
benefit from an effect of local of minocycline. Subgingival debridement in
combination with oral hygiene instruction by itself has been shown to be
effective. It remains to be studied whether local application of
minocycline can be effective as an adjunct to mechanical therapy in sites
that respond poorly to conventional treatment.
=========================================================================
28.) Clinical and microbiological effects of minocycline-loaded
microcapsules in adult periodontitis.
=========================================================================
Author
Yeom HR; Park YJ; Lee SJ; Rhyu IC; Chung CP; Nisengard RJ
Address
Department of Periodontology, College of Dentistry, Seoul National
University, Korea.
Source
J Periodontol, 68(11):1102-9 1997 Nov
Abstract
Clinical and microbiological effects of subgingival delivery of 10%
minocycline-loaded (MC), bioabsorbable microcapsules were examined in 15
adult periodontitis patients. Patients received oral hygiene instruction 2
weeks prior to the study. At baseline (day 0) all teeth received
supragingival scaling (SC); 2 quadrants received no further treatment and 1
quadrant received subgingival scaling and root planning (SRP). In the
fourth quadrant, the tooth with the deepest probing sites (at least 1 site
> or = 5 mm) was treated with minocycline microcapsules. The sites were
evaluated at baseline and weeks 1, 2, 4, and 6. Clinical indices included
bleeding on probing (BOP), probing depths (PD), and attachment loss (AL).
Microbiological evaluations included percent morphotypes by phase-contrast
microscopy; cultivable anaerobic, aerobic, and black-pigmented Bacteroides
(BPB); and percent Porphyromonas gingivalis, Prevotella intermedia,
Eikenella corrodens, and Actinomyces viscosus by indirect
immunofluorescence. In the SC + MC group, BOP, PD, and AL were
significantly reduced from baseline for weeks 1 to 6. BOP in the SC + MC
group was significantly reduced compared to the SRP group from weeks 2 to
6. In the SC + MC group the percent of spirochetes and motile rods
decreased and the percent of cocci increased after 1 week. The increased
cocci and decreased motile rods were statistically greater at weeks 4 and 6
in the SC + MC group compared to the SRP group. This study demonstrates
that local subgingival delivery of 10% minocycline-loaded microcapsules as
an adjunct to scaling results in reduction in the percent sites bleeding on
probing greater than scaling and root planning alone and induces a
microbial response more favorable for periodontal health than scaling and
root planing.
=========================================================================
29.) The broad-spectrum activity and efficacy of catheters coated with
minocycline and rifampin.
=========================================================================
Author
Raad I; Darouiche R; Hachem R; Mansouri M; Bodey GP
Address
Section of Infectious Diseases, University of Texas M. D. Anderson Cancer
Center, Houston 77030, USA.
Source
J Infect Dis, 173(2):418-24 1996 Feb
Abstract
The in vitro and in vivo activities of catheters coated with minocycline
and rifampin and with chlorhexidine gluconate and silver sulfadiazine were
evaluated. When incubated in serum at 37 degrees C, the half-life of the
inhibitory activity of catheters coated with minocycline and rifampin was
25 days compared with 3 days for catheters coated with chlorhexidine
gluconate and silver sulfadiazine. In a rabbit model, catheters coated with
minocycline and rifampin were significantly more efficacious than catheters
coated with chlorhexidine and silver sulfadiazine in preventing
colonization and infection with Staphylococcus aureus (P < .05). Catheters
coated with minocycline and rifampin demonstrated broad-spectrum in vitro
inhibitory activity against gram-positive bacteria, gram-negative bacteria,
and Candida albicans that was significantly superior to the inhibitory
activity of catheters coated with chlorhexidine gluconate and silver
sulfadiazine (P < .01). Minocycline and rifampin were also highly
efficacious in preventing colonization and infection in vivo.
=========================================================================
30.) Symptomatic hepatic cysts: treatment with single-shot injection of
minocycline hydrochloride.
=========================================================================
Author
Cellier C; Cuenod CA; Deslandes P; Auroux J; Landi B; Siauve N; Barbier JP;
Frija G
Address
Department of Gastroenterology, Universit´e Ren´e Descartes, H^opital
Laennec, Paris, France.
Source
Radiology, 206(1):205-9 1998 Jan
Abstract
PURPOSE: To assess the efficacy of percutaneous minocycline hydrochloride
sclerotherapy in symptomatic hepatic cysts. MATERIALS AND METHODS: From
November 1992 to June 1994, seven of eight consecutive adults with large
symptomatic hepatic cysts (diameter, 55-130 mm) were treated with a single
intracystic injection of minocycline hydrochloride in an ambulatory
procedure. Five patients had a solitary cyst, and two had polycystic liver
disease. The target cyst was punctured under ultrasound guidance and local
anesthesia with a 22-gauge Chiba needle. Half of the cyst content was
aspirated before injection of 100-500 mg of minocycline hydrochloride
diluted in 5-25 mL of saline. The minocycline hydrochloride was left in the
cyst at the end of the procedure. RESULTS: After a mean follow-up of 28
months (range, 24-42 months), all five patients with solitary cysts were
asymptomatic and four had documented complete cyst regression; the two
patients with multiple hepatic cysts showed only transient clinical
improvement. CONCLUSION: Single-shot injection of minocycline hydrochloride
is an effective treatment for symptomatic solitary hepatic cysts but is
less effective in polycystic liver disease.
=========================================================================
()()()()()()()()()()( LO MALO () THE BAD ()()()()()()()()()()()()()()()()
=========================================================================
31.) Minocycline induced autoimmune hepatitis and systemic lupus
erythematosus-like syndrome [see comments]
=========================================================================
Author
Gough A; Chapman S; Wagstaff K; Emery P; Elias E
Address
Department of Rheumatology, Harrogate District Hospital.
Source
BMJ, 312(7024):169-72 1996 Jan 20
Abstract
Monocycline is the most widely prescribed systemic antibiotic for acne
largely because it needs to be given only once or twice a day and seems not
to induce resistance. Up to April 1994 11 cases of minocycline induced
systemic lupus erythematosus and 16 cases of hepatitis had been reported to
the Committee on Safety of Medicines. An analysis of these cases together
with seven other cases shows the severity of some of these reactions. Two
patients died while taking the drug for acne and a further patient needed a
liver transplant. Acne itself can induce arthritis and is often seen in
association with autoimmine liver disease, but the clinical and biochemical
resolution seen after withdrawal of the drug, despite deterioration of the
acne, suggests a drug reaction. In five cases re-exposure led to
recurrence. Because reactions may be severe early recognition is important
to aid recovery and also to avoid invasive investigations and treatments
such as corticosteroids and immunosuppresants. Safer alternatives should be
considered for treating acne.
=========================================================================
32.) Minocycline and Lupuslike Syndrome in Acne Patients
=========================================================================
Miriam C. J. M. Sturkenboom, PhD, PharmD, MSc; Christoph R. Meier, PhD,
MSc; Hershel Jick, MD; Bruno H. C. Stricker, PhD, MB
Arch Intern Med. 1999;159:493-497
Background: Recently several case reports described the association between
minocycline and lupuslike syndrome. Minocycline, one of
the tetracyclines, is widely used to treat acne. We aimed to examine the
association of exposure to minocycline and other tetracyclines with the
development
of lupuslike syndrome.
Methods: We conducted a nested case-control study in a cohort of 27,688 acne
patients aged 15 to 29 years, using data automatically recorded on general
practitioners' office computers in the United Kingdom. Controls were matched
to cases on age, sex, and practice. The main outcome was lupuslike syndrome
defined as the occurrence of polyarthritis or polyarthralgia of unknown
origin,
with negative rheumatoid factor or latex agglutination test, positive or
unmeasured antinuclear factor, elevated or unmeasured erythrocyte
sedimentation rate, and absence of or unmeasured antinative DNA antibody
levels.
Results: We identified 29 cases and selected 152 controls. Current single
use of
minocycline was associated with an 8.5-fold (95% confidence interval [CI],
2.1-35) increased risk of developing lupuslike syndrome compared with
nonusers and past users of tetracyclines combined. The risk of past
exposure to
any of the tetracyclines was closely similar to nonuse (relative risk, 1.3;
95%
CI, 0.5-3.3). Current use of doxycycline, oxytetracycline, or tetracycline
combined was associated with a 1.7-fold (95% CI, 0.4-8.1) increase of risk.
The
risk increased with longer use.
Conclusion: Current use of minocycline increased the risk of developing
lupuslike syndrome 8.5-fold in the cohort of young acne patients. The
effect was
stronger in longer-term users. However, the absolute risk of developing
lupuslike syndrome seems to be relatively low.
=========================================================================
33.) Minocycline-induced lupus.
=========================================================================
Author
Farver DK
Address
College of Pharmacy, South Dakota State University, Brookings, USA.
dfarver@sunflowr.usd.edu
Source
Ann Pharmacother, 31(10):1160-3 1997 Oct
Abstract
OBJECTIVE: To report a case of drug-induced lupus occurring 5 months after
the initiation of minocycline therapy for acne. DATA SOURCE: Case report
information was obtained from the physician, patient's family, and the
medical record. MEDLINE and Index Medicus were searched to obtain relevant
published literature from 1966 to 1996. CASE SUMMARY: A 14-year-old white
girl developed symptoms of myalgias, arthralgias, polyarthritis, and
flushed face. The antinuclear antibody test was positive. Minocycline was
discontinued and the patient's condition dramatically improved within 7
days. CONCLUSIONS: Healthcare providers should recognize early common and
unusual symptoms of minocycline-induced lupus in adolescents being treated
for acne.
=========================================================================
34.) Minocycline related lupus.
=========================================================================
Author
Masson C; Chevailler A; Pascaretti C; Legrand E; Br´egeon C; Audran M
Address
Service de Rhumatologie, CHU d'Angers, France.
Source
J Rheumatol, 23(12):2160-1 1996 Dec
Abstract
The determination of a factor triggering lupus-like symptoms could yield
new insights into the management of rheumatic disease. We describe a case
of minocycline related lupus in a young patient positive for HLA-DR2 who
was prescribed minocycline 4 times for mild acne and developed rheumatic
symptoms each time. We review 8 other cases.
=========================================================================
35.) Acute hepatitis and drug-related lupus induced by minocycline treatment.
=========================================================================
Author
Golstein PE; Deviere J; Cremer M
Address
Department of Gastroenterology, Erasmus Hospital, Universit´e Libre de
Bruxelles, Belgium.
Source
Am J Gastroenterol, 92(1):143-6 1997 Jan
Abstract
Minocycline is widely prescribed for long-term treatment in acne. Major
side effects are rare and include hepatitis and drug-related lupus.
Hepatitis can be early and acute or late and chronic, whereas lupus
presents as a tardive and insidious disease. We report a case of
minocycline-induced lupus initially presenting as acute hepatitis, evolving
to chronic cytolysis, in a young man treated for facial acne.
=========================================================================
36.) Minocycline-induced pneumonitis with bilateral hilar lymphadenopathy
and pleural effusion.
=========================================================================
ARTICLE SOURCE: Intern Med (Japan), Mar 1994, 33(3) p177-9
AUTHOR(S): Bando T; Fujimura M; Noda Y; Hirose J; Ohta G; Matsuda T
PUBLICATION TYPE: JOURNAL ARTICLE; REVIEW (12 references); REVIEW,
ABSTRACT: A 65-year-old man developed respiratory failure with diffuse
interstitial shadow, bilateral pleural effusion, and bilateral hilar
lymphadenopathy on chest X-ray and CT, after intravenous administration of
minocycline. Corticosteroid therapy was effective. The findings from
bronchoalveolar lavage (BAL) and transbronchial lung biopsy were compatible
with eosinophilic pneumonia. Provocation test supported this diagnosis, but
the lymphocyte stimulation test was negative. A review of the literature
and the diagnoses of drug-induced pulmonary diseases are discussed.
=========================================================================
37.) Comparative safety of tetracycline, minocycline, and doxycycline.
=========================================================================
Author
Shapiro LE; Knowles SR; Shear NH
Address
Department of Medicine, Sunnybrook Hospital, University of Toronto Medical
School, Ontario, Canada.
Source
Arch Dermatol, 133(10):1224-30 1997 Oct
Abstract
BACKGROUND: Because minocycline can cause serious adverse events including
hypersensitivity syndrome reaction (HSR), serum sicknesslike reaction
(SSLR), and drug-induced lupus, a follow-up study based on a retrospective
review of our Drug Safety Clinic and the Health Protection Branch databases
and a literature review was conducted to determine if similar rare events
are associated with tetracycline and doxycycline. Cases of isolated single
organ dysfunction (SOD) attributable to the use of these antibiotics also
were identified. OBSERVATIONS: Nineteen cases of HSR due to minocycline, 2
due to tetracycline, and 1 due to doxycycline were identified. Eleven cases
of SSLR due to minocycline, 3 due to tetracycline, and 2 due to doxycycline
were identified. All 33 cases of drug-induced lupus were attributable to
minocycline. Forty cases of SOD from minocycline, 37 cases from
tetracycline, and 6 from doxycycline were detected. Hypersensitivity
syndrome reaction, SSLR, and SOD occur on average within 4 weeks of
therapy, whereas minocycline-induced lupus occurs on average 2 years after
the initiation of therapy. CONCLUSIONS: Early serious events occurring
during the course of tetracycline antibiotic treatment include HSR, SSLR,
and SOD. Drug-induced lupus, which occurs late in the course of therapy, is
reported only with minocycline. We theorize that minocycline metabolism may
account for the increased frequency of serious adverse events with this drug.
=========================================================================
38.) Serious adverse reactions induced by minocycline. Report of 13
patients and review of the literature.
=========================================================================
Author
Knowles SR; Shapiro L; Shear NH
Address
Division of Clinical Pharmacology, Sunnybrook Health Science Centre,
Toronto, Ontario.
Source
Arch Dermatol, 132(8):934-9 1996 Aug
Abstract
BACKGROUND: Minocycline has been reported to cause serious, albeit rare,
adverse events, including serum sickness-like reaction, hypersensitivity
syndrome reaction, and drug-induced lupus. A retrospective review of
patients seen in our Adverse Drug Reaction Clinic as well as information
obtained from the Health Protection Branch was done to identify patients
with minocycline-induced reactions. In addition, the literature concerning
serious reactions to minocycline was reviewed. OBSERVATIONS: Six patients
with a hypersensitivity syndrome reaction, 6 patients with a serum
sickness-like reaction, and 1 patient who had symptoms consistent with
drug-induced lupus were identified. A review of the literature identified
11 cases of hypersensitivity syndrome reaction, 1 case of serum
sickness-like reaction, and 24 cases of drug-induced lupus. Serum
sickness-like reactions occur sooner than hypersensitivity syndrome
reactions (15.6 vs 23.7 days, P = .04). Drug-induced lupus occurs on
average 2 years after the start of minocycline therapy. CONCLUSIONS:
Dermatologists need to be aware of the serious adverse reactions that can
develop after minocycline use. In patients who may require long-term
therapy with minocycline ( > 1 year), we suggest that antinuclear antibody
and hepatic transaminase levels be determined at baseline. Rechallenge with
minocycline or other tetracyclines is currently not recommended for
patients who develop these serious reactions.
Language
=========================================================================
39.) [Side effects of minocycline in the treatment of acne vulgaris]
=========================================================================
Author
Hoefnagel JJ; van Leeuwen RL; Mattie H; Bastiaens MT
Address
Afd. Dermatologie, Leids Universitair Medisch Centrum, Leiden.
Source
Ned Tijdschr Geneeskd, 141(29):1424-7 1997 Jul 19
Abstract
Minocycline is the most commonly used systemic antibiotic in the long-term
treatment (weeks to months) of severe acne vulgaris. Currently much
attention is being paid in the Dutch and international literature to the
safety of minocycline, after several reports on serious adverse events. The
clinical efficacy of minocycline in the treatment of acne vulgaris is
better than that of tetracycline and equal to that of doxycycline. The
serious adverse events of minocycline therapy described consist of
hyperpigmentation of various tissues, autoimmune disorders (systemic lupus
erythematosus, autoimmune hepatitis) and serious hypersensitivity reactions
(hypersensitivity syndrome reaction, pneumonitis and eosinophilia, and
serum sickness-like syndrome). In relation to the number of prescriptions,
the number of serious adverse events of minocycline described is small.
However, it is very important that prescribing doctors should be aware of
the possibility of these adverse events occurring during long-term
minocycline therapy and able to recognize the characteristic symptoms at an
early stage.
=========================================================================
40.) Serious dermatologic reactions in children.
=========================================================================
Author
Knowles S; Shapiro L; Shear NH
Address
Department of Clinical Pharmacology, Sunnybrook Health Science Centre,
Toronto, Ontario, Canada.
Source
Curr Opin Pediatr, 9(4):388-95 1997 Aug
Abstract
Although serious reactions comprise only a small percentage of total
adverse drug reactions, they are important in terms of morbidity and
potential mortality. An update on serious dermatologic reactions in
children is presented including serum sickness-like reactions due to
cefaclor, hypersensitivity syndrome reactions (HSRs), and drug-induced
pseudoporphyria. More detailed information on minocycline-induced reactions
including drug-induced lupus and HSRs and lamotrigine-induced toxic
epidermal necrolysis and Stevens-Johnson syndrome will be discussed.
=========================================================================
41.) Serum sickness-like syndrome associated with minocycline therapy.
=========================================================================
ARTICLE SOURCE: Allergy (Denmark), May 1990, 45(4) p313-5
AUTHOR(S): Puyana J; Urena V; Quirce S; Fernandez-Rivas M; Cuevas M; Fraj J
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: A 19 year-old youth was taking oral minocycline and after 8 days
he presented all four cardinal symptoms of serum sickness (urticaria,
fever, lymphadenopathy and joint symptoms). C3, C4 and CH50 evolution
imitate experimental serum sickness complement evolution. We exclude other
causes of this syndrome. Although other hypersensitivity reactions have
occurred with minocycline usage, to our knowledge serum sickness-like
syndrome has not been previously reported with this drug.
=========================================================================
42.) Minocycline-induced loss of potassium from erythrocytes:
identification of a family with an augmented response.
=========================================================================
ARTICLE SOURCE: J Infect Dis (United States), Oct 1978, 138(4) p455-62
AUTHOR(S): Kornguth ML; Kunin CM
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: The effect of several tetracycline antibiotics of human
erythrocytes was examined because of previous findings that these drugs
bind to erythrocyte membranes. Minocycline and cetocycline, two highly
lipid-soluble analogues, but not tetracycline, induced loss of K+ from red
blood cells. Loss of K+ increased linearly with time of incubation,
concentration of minocycline, and temperature. The effect of minocycline
was inhibited by plasma and calcium. The cells from one volunteer
consistently showed an augmented response to minocycline; similar findings
for family members of the volunteer suggested a dominant autosomal mode of
inheritance. The only abnormality noted in the subject was mild
reticulocytosis and a slightly reduced K+ content in his red blood cells.
Preliminary studies did not demonstrate alterations in protein composition
of his red blood cell membranes, enhanced osmotic fragility, or defects in
Ca++-dependent or ouabain-sensitive (Na+-K+)-dependent adenosine
triphosphatase activity. The exact site of the minocycline effect remains
to be determined.
=========================================================================
()()()()()()()()()()()()()() LO FEO () THE UGLY ()()()()()()()()()()()()
=========================================================================
43.) Minocycline-induced hyperpigmentation in leprosy.
=========================================================================
Author
Fleming CJ; Hunt MJ; Salisbury EL; McCarthy SW; Barnetson RS
Address
Department of Dermatology, Royal Prince Alfred Hospital, New South Wales,
Australia.
Source
Br J Dermatol, 134(4):784-7 1996 Apr
Abstract
A 36-year-old man was treated with dapsone, rifampicin and clofazimine for
borderline lepromatous leprosy. After 9 months, his leprosy plaques became
progressively more red and after 23 months, the clofazimine was stopped and
he was given minocycline instead. Six weeks later, he developed blue-black
pigmentation in his leprosy lesions. The histology was consistent with
minocycline-induced hyperpigmentation. This is the first report of
minocycline-induced pigmentation in leprosy. We suggest it is important to
consider this side-effect before the administration of minocycline in
leprosy, particularly if it is prescribed in place of clofazimine.
=========================================================================
44.) Psoriatic arthritis and minocycline induced autoantibodies.
=========================================================================
Leitch DN; Haslock DI
Department of Rheumatology, South Cleveland Hospital, Middlesbrough,
Cleveland.
Clin Rheumatol (BELGIUM) May 1997 16 (3) p317-8 ISSN: 0770-3198
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9710
Subfile: INDEX MEDICUS
A case of psoriatic arthritis where diagnosis was originally complicated
by the
presence of minocycline-induced auto-antibodies and hepatic dysfunction.
The range
of auto-antibodies associated with minocycline includes ds DNA and SCL 70.
=========================================================================
45.) Minocycline hydrochloride hyperpigmentation complicating treatment of
pyoderma gangrenosum.
=========================================================================
ARTICLE SOURCE: J Dermatol (Japan), Dec 1994, 21(12) p965-7
AUTHOR(S): Miralles ES; Nunez M; Perez B; Ledo A
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Minocycline-associated hyperpigmentation is an uncommon side
effect. We report the case of a patient with pyoderma gangrenosum
successfully treated with oral minocycline but complicated by marked
hyperpigmentation in his pyoderma gangrenosum and acne scars. One of the
clinical forms of minocycline hyperpigmentation includes dark-blue or black
macules in depressed acne scars or other sites of skin inflammation; this
pattern seems to be independent of the total cumulative dose and the skin
process.
=========================================================================
46.) Minocycline-induced oral pigmentation.
=========================================================================
ARTICLE SOURCE: J Am Acad Dermatol (United States), Feb 1994, 30(2 Pt 2)
p350-4
AUTHOR(S): Siller GM; Tod MA; Savage NW
PUBLICATION TYPE: JOURNAL ARTICLE
ABSTRACT: Oral mucosal pigmentation is an infrequently reported side
effect of minocycline. Two patients with minocycline deposition within
teeth and bone, demonstrated by fluorescence microscopy, are described.
Minocycline is the only tetracycline reported to cause discoloration of the
oral mucosa. This may be the result of deposition of an insoluble
degradation product of minocycline in the underlying bone. Pigmentation is
not necessarily dose-dependent and may take months or years to resolve.
=========================================================================
47.) Nodular hyperplasia, black thyroid, and chronic minocycline ingestion
in a teenager.
=========================================================================
ARTICLE SOURCE: Arch Surg (United States), Dec 1992, 127(12) p1476-7
AUTHOR(S): Folsom DL; Gauderer MW; Dahms WT
PUBLICATION TYPE: JOURNAL ARTICLE; REVIEW (7 references); REVIEW OF
REPORTED CASES
ABSTRACT: An 18-year-old man with left-lobe thyroid hemiagenesis underwent
isthmectomy for management of a nodule that failed to take up radioactive
iodine during a nuclear scan. The resected tissue, which demonstrated
nodular hyperplasia, and the remaining right lobe, were black. The
association between deep staining and chronic minocycline ingestion was
subsequently recognized. Twelve years later, the patient remained
asymptomatic, suggesting that complete resection of tetracycline-stained
thyroid tissue is unnecessary.
=========================================================================
48.) Black bones following long-term minocycline treatment.
=========================================================================
ARTICLE SOURCE: Arch Pathol Lab Med (United States), Sep 1991, 115(9)
p939-41
AUTHOR(S): Rumbak MJ; Pitcock JA; Palmieri GM; Robertson JT
PUBLICATION TYPE: JOURNAL ARTICLE; REVIEW (16 references); REVIEW OF
REPORTED CASES
ABSTRACT: During a surgical procedure, black vertebrae were observed in a
42-year-old white woman. An undecalcified iliac crest bone biopsy specimen
revealed intense fluorescence compatible with tetracycline labeling and
osteoporosis. A urinary screening test was negative for amino acids. The
patient had been treated with minocycline hydrochloride (100 to 300 mg/d)
for at least 6 years. Since minocycline is known to discolor many body
tissues, it is likely that the black discoloration of bone in our patient
was caused by the long-term intake of the antibiotic.
=========================================================================
49.) Pigmented postacne osteoma cutis in a patient treated with
minocycline: report and review of the literature.
=========================================================================
ARTICLE SOURCE: J Am Acad Dermatol (United States), May 1991, 24(5 Pt 2)
p851-3
AUTHOR(S): Moritz DL; Elewski B
PUBLICATION TYPE: JOURNAL ARTICLE; REVIEW (19 references); REVIEW, TUTORIAL
ABSTRACT: Postacne osteoma cutis is a rare complication of acne vulgaris.
If it occurs during a course of tetracycline or minocycline therapy,
pigmented osteomas can occur as a result of tetracycline or minocycline
bone complexes. We report a case of pigmented postacne osteoma cutis that
developed after extensive acne surgery and a 2- to 3-month course of
minocycline. Previously reported cases have been treated surgically, but
our patient responded to 0.05% tretinoin cream, with transepidermal
elimination of some osteomas.
=====================================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No (45) 17/03/99 DR. JOSE LAPENTA R.
======================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela 1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo
Venezuela
1.998-2.024
Tlf: 0414-2976087 - 04127766810














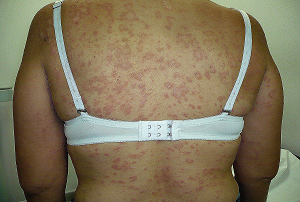




.png)