REVISTA DERMATOLÓGICA AGOSTO 2003-2024, DUTASTERIDE, AVODART
En este bloque de publicaciones del año 2003, destaca LA MEDICINA DUTASTERIDE (9,10), lanzada al mercado en el año 2004, es decir 7 (siete) años después de la APROBACIÓN DEL FINASTERIDE lo cual ocurrió en 1998.
Sin embargo el DUTASTERIDE utiliza igual que este último (FINASTERIDE), para la Hiperplasia prostática benigna (HPB), y la alopecia androgénica, ya que es un inhibidor de la enzima 5-alfa reductasa, la cual inhibe la producción de DIHIDROTESTOSTERONA (DHT), metabolito responsable de estas dos patologías.
DIFERENCIAS ENTRE FINASTERIDE Y DUTASTERIDE:
A.-FINASTERIDE:
1.) El FINASTERIDE, inhibe solo la enzima 5-alfa reductasa II, con un 70% de efectividad en la Hiperplasia prostática benigna (HPB).
2.) El FINASTERIDE esta aprobado tanto para esta adicción (HPB), como para el tratamiento de la ALOPECIA ANDROGÉNICA.
3.) El FINASTERIDE no tiene presentación en ampollas para tratar el alopecia androgénica con micro inyecciones en el cuero cabelludo.
4.) El FINASTERIDE se esta utilizando en CREMA para el tratamiento del hirsutismo en la mujer.
5.) Se ha descrito el SÍNDROME POST FINASTERIDE que se presenta luego de abandonar el tratamiento prolongado con esta medicina, tanto para hiperplasia prostática, como alopecia androgénica.
B.-DUTASTERIDE:
1.) El DUTASTERIDE inhibe las enzimas 5.alfa reductasas I y II, con un supuesto mayor efecto (95%) en la Hiperplasia Prostática benigna (HPB).
2.) El DUTASTERIDE NO ESTÁ OFICIALMENTE APROBADO para el tratamiento de LA ALOPECIA ANDROGÉNICA, en algunos países si esta aprobado , como COREA, donde se aprobó en el 2009, para tratar esta afección.
3.) Sin embargo. el DUTASTERIDE se está utilizando en presentación de AMPOLLAS con micro inyecciones en el cuero cabelludo para tratar la alopecia androgénica.
4.) El DUTASTERIDE NO se utiliza para el tratamiento del HIRSUTISMO, en la mujer.
5.) NO ESTÁ BIEN DOCUMENTADO el síndrome post suspensión del DUTASTERIDE, luego de tratamientos prolongados, pero se cree que por ser moléculas similares, pudiera producirlo.
Enlace sobre publicación del FINASTERIDE II y sus efectos.
Otros temas interesantes: La aprobación del VALTREX (valaciclovir) para prevenir la transmisión de enfermedades de transmisión sexual (ETS), sobre todo el herpes simple tipo II. (1)
Una investigación del Roacutan (ISOTRETINOINA) (2, 3, 4, 5), como agente causal de SUICIDIOS, lo cual ya documente en la publicación LA ISOTRETINOINA, LO BUENO, LO MALO Y LO FEO, CLICK.
Otros Efectos adversos del ORLISTAT (XENICAL), (6,7). también ya publicado en este enlace sobre LOS EFECTOS ADVERSOS DEL ORLISTAT.
NIMESULIDE vinculado a muertes en la INDIA (8), que encuentras también en este enlace sobre EL NIMESULIDE, UNA HISTORIA PARA REFLEXIONAR.
Sinopsis de los síndromes hereditarios de las poikilo dermatosis (11).
Saludos,,,
Dr. José Lapenta.
ENGLISH
In this block of publications from the year 2003, the DUTASTERIDE MEDICINE (9,10) stands out, launched on the market in 2004, that is, 7 (seven) years after the APPROVAL OF FINASTERIDE, which occurred in 1998.
However, DUTASTERIDE is used the same as the latter (FINASTERIDE), for benign prostatic hyperplasia (BPH), and androgenic alopecia, since it is an inhibitor of the enzyme 5-alpha reductase, which inhibits the production of DIHYDROTESTOSTERONE (DHT), the metabolite responsible for these two pathologies.
DIFFERENCES BETWEEN FINASTERIDE AND DUTASTERIDE:
A.-FINASTERIDE:
1.) FINASTERIDE inhibits only the enzyme 5-alpha reductase II, with 70% effectiveness in benign prostatic hyperplasia (BPH).
2.) FINASTERIDE is approved for this addiction (BPH), as well as for the treatment of ANDROGENETIC ALOPECIA.
3.) FINASTERIDE is not available in ampoules to treat androgenic alopecia with micro injections in the scalp.
4.) FINASTERIDE is being used in CREAM for the treatment of hirsutism in women.
5.) POST-FINASTERIDE SYNDROME has been described, which occurs after stopping prolonged treatment with this medicine, both for prostatic hyperplasia and androgenic alopecia.
B.-DUTASTERIDE:
1.) DUTASTERIDE inhibits the enzymes 5-alpha reductases I and II, with a supposed greater effect (95%) in benign prostatic hyperplasia (BPH).
2.) DUTASTERIDE IS NOT OFFICIALLY APPROVED for the treatment of ANDROGENIC ALOPECIA, in some countries it is approved, such as KOREA, where it was approved in 2009, to treat this condition.
3.) However, DUTASTERIDE is being used in the form of AMPOULES with micro injections in the scalp to treat androgenic alopecia.
4.) DUTASTERIDE is NOT used for the treatment of HIRSUTISM in women.
5.) Post-discontinuation syndrome of DUTASTERIDE is NOT WELL DOCUMENTED, after prolonged treatments, but it is believed that because they are similar molecules, it could cause it.
Link to publication of FINASTERIDE II and its effects.
Other interesting topics: The approval of VALTREX (valacyclovir) to prevent the transmission of sexually transmitted diseases (STDs), especially herpes simplex type II. (1)
A research on Accutane (ISOTRETINOIN) (2, 3, 4, 5), as a causal agent of SUICIDES, which I already documented in the publication ISOTRETINOIN, THE GOOD, THE BAD AND THE UGLY, CLICK.
Other adverse effects of ORLISTAT (XENICAL), (6,7). also already published in this link on THE SIDE EFFECTS OF ORLISTAT.
NIMESULIDE linked to deaths in INDIA (8), which you can also find in this link on NIMESULIDE, A HISTORY TO REFLECT ON.
Synopsis of the hereditary syndromes of poikilodermatous (11).
Greetings...
Dr. José Lapenta R.
VALACYCLOVIR, ISOTRETINOIN, NIMESULIDE, ORLISTAT, DUTASTERIDE, HEREDITARY POIKILODERMATOUS SYNDROMES
1.) FDA Approves Valtrex for Reducing Risk For Sexual Transmission With Suppressive Therapy.
2.) Investigan droga contra el acne (Roaccutan) en casos de depresión y suicidios.
3.) Mother of Suicidal Tampa Pilot Says She Will Sue Maker of Accutane
4.) Mother of 15-year-old Tampa suicide pilot blaming acne drug for boy's death.(Accutane)/ 147 intentos de suicidio reporta FDA !!!
7.) Orlistat e hipertensión / Xenical e hipertensión.
9.) WHAT IS DUTASTERIDE (AVODART™ AVOLVE™) /Nueva medicina para la caida del cabello
10.) AVODART™ / DUTASTERIDE/ The Biggest News In Hair Loss Treatment Since Propecia
11.) Synopsis of Hereditary Poikilodermatous syndromes
FDA Updates Labeling of Valtrex source: Aug 29, 2003
FDA Talk Paper
Source: http://www.natap.org/
The Food and Drug Administration (FDA) has approved a new
indication for Valtrex (valacyclovir hydrochloride)
Caplets; Valtrex reduces the risk of heterosexual
transmission of genital herpes to susceptible partners
with healthy immune systems when used as suppressive
therapy in combination with safer sex practices.
Genital herpes is a common sexually transmitted infection
caused by herpes simplex viruses (HSV). Many individuals
have no or only minimal signs or symptoms from genital HSV
infection and may transmit the virus during sexual contact
when they show no signs of active infection (i.e. genital
lesions).
The following safer sex practices can also lower the
chances of passing genital herpes to a partner:
--Use a condom made of latex or polyurethane when you have
sexual contact. --Do not have sexual contact with your
partner when you have any symptom or outbreak of genital
herpes.
FDA based its decision to revise the labeling for Valtrex
on the results of an international, double-blind,
placebo-controlled clinical trial conducted by the
manufacturer, GlaxoSmithKline (GSK) of Research Triangle
Park, N.C. The study was conducted among about 1500
monogamous, heterosexual couples and lasted eight months.
At the beginning of the study, only one member in each
couple had evidence of genital herpes. The results of the
GSK study showed a 48% reduction in HSV acquisition;
individual results may vary based on consistency of safer
sex practices.
Valtrex may cause kidney problems in some people. In
addition, Valtrex may cause nervous system problems; these
include aggressive behavior, unsteady movements, shaky
movements, confusion, speech problems, hallucinations,
seizures and coma. Kidney and nervous system problems have
happened in patients who already have kidney disease and
in elderly patients whose kidneys do not work well due to
age. Therefore, it is important for patients to tell their
healthcare providers if they have kidney problems or other
medical conditions before taking Valtrex.
Today’s action follows the recommendation of FDA’s
Antiviral Drugs Advisory Committee, which met on May 14,
2003, to discuss GSK’s then-proposed use of Valtrex for
reduction of the risk of transmission of genital herpes
with the use of suppressive therapy. FDA first approved
Valtrex in 1995.
To read report on FDA Hearing and review of study results:
Valtrex For Reducing Transmission of Genital Herpes- FDA
Hearing: FDA panel votes 11-0 to recommend approval
http://www.natap.org/2003/may/051503_1.htm
CLINICAL TRIALS Herpes Zoster: Two randomized double-blind
clinical trials in immunocompetent adults with localized
herpes zoster were conducted. VALTREX was compared to
placebo in patients less than 50 years of age, and to
ZOVIRAX in patients greater than 50 years of age. All
patients were treated within 72 hours of appearance of
zoster rash. In patients less than 50 years of age, the
median time to cessation of new lesion formation was 2
days for those treated with VALTREX compared to 3 days for
those treated with placebo. In patients greater than 50
years of age, the median time to cessation of new lesions
was 3 days in patients treated with either VALTREX or
ZOVIRAX. In patients less than 50 years of age, no
difference was found with respect to the duration of pain
after healing (post-herpetic neuralgia) between the
recipients of VALTREX and placebo. In patients greater
than 50 years of age, among the 83% who reported pain
after healing (post-herpetic neuralgia), the median
duration of pain after healing [95% confidence interval]
in days was: 40 [31, 51, 43 [36, 55], and 59 [41, 77] for
7-day VALTREX, 14-day VALTREX, and 7-day ZOVIRAX,
respectively.
Genital Herpes Infections: Initial Episode: Six hundred
and forty-three immunocompetent adults with first episode
genital herpes who presented within 72 hours of symptom
onset were randomized in a double-blind trial to receive
10 days of VALTREX 1 gram twice daily (n = 323) or ZOVIRAX
200 mg 5 times a day (n = 320). For both treatment groups:
the median time to lesion healing was 9 days, the median
time to cessation of pain was 5 days, the median time to
cessation of viral shedding was 3 days.
Recurrent Episodes: Three double-blind trials (2 of them
placebo-controlled) in immunocompetent adults with
recurrent genital herpes were conducted. Patients
self-initiated therapy within 24 hours of the first sign
or symptom of a recurrent genital herpes episode. In 1
study, patients were randomized to receive 5 days of
treatment with either VALTREX 500 mg twice daily (n = 360)
or placebo (n = 259). The median time to lesion healing
was 4 days in the group receiving VALTREX 500 mg versus 6
days in the placebo group, and the median time to
cessation of viral shedding in patients with at least 1
positive culture (42% of the overall study population) was
2 days in the group receiving VALTREX 500 mg versus 4 days
in the placebo group. The median time to cessation of pain
was 3 days in the group receiving VALTREX 500 mg versus 4
days in the placebo group. Results supporting efficacy
were replicated in a second trial.
In a third study, patients were randomized to receive
VALTREX 500 mg twice daily for 5 days (n = 398) or VALTREX
500 mg twice daily for 3 days (and matching placebo twice
daily for 2 additional days) (n = 402). The median time to
lesion healing was about 4.5 days in both treatment
groups. The median time to cessation of pain was about 3
days in both treatment groups.
![]()
2.) Investigan droga contra el acne (Roaccutan) en
casos de depresion y suicidios
El medicamento se comercializa en Chile bajo receta medica retenida
Source: http://www.geocities.com/econatsalud/noticias/
Pedro Vicario
Caso del joven norteamericano que estrello su avioneta
contra un edificio en EE.UU., quien se presume consumía el
medicamento, revivio dudas contra el fármaco.
Charles Bishop, el adolescente de 15 años que el pasado sábado -emulando los atentados al World Trade Center- estrelló un avión Cessna contra el edificio del Bank of America Plaza en Tampa, Florida, era un joven normal. Tan normal que tenía los problemas propios de los jóvenes de su edad. En el hogar del novel piloto fue encontrada una receta prescrita a su nombre con el fármaco Accutane, versión norteamericana del medicamento vendido en Chile Roacnetan, que se usa comúnmente para el tratamiento del acné juvenil.
El hecho volvió a sacar al tapete el tema de los riesgos que esta droga pudiera tener en la población. A las ya conocidas contraindicaciones a embarazadas por riesgo de malformaciones y muertes de sus hijos (advertidas debidamente en su envase), se agrega la sospecha de que el fármaco pudiera ocasionar depresión e incluso intentos de suicidio. Ese podría ser el caso del joven kamikaze norteamericano quien, a juzgar por la receta encontrada en su casa, era un típico consumidor de la droga.
El Accutane -su nombre genérico es isotretinoína- se vende en Chile con receta médica retenida bajo el nombre de Roacnetan. El laboratorio Roche, productor del fármaco, aclaró a través de la vicepresidenta del laboratorio farmacéutico Hoffman-La Roche, Carolyn Glynn, que la empresa estaba tomando el caso “muy seriamente”. “Ciertamente que vamos a analizar este caso detenidamente” agregó Glynn, ante la magnitud que pudiera tomar el problema, tomando en cuenta que el medicamento es recetado a pacientes de todo el mundo.
¿Qué pasa en Chile?
Según el psiquiatra José Bitrán, el ha tenido dudas respecto de pacientes puntuales que han tomado el Roacnetan. “El paciente de uno que tiene problemas anímicos y a su vez es un adolescente, en la mitad del tratamiento empieza a tomar el fármaco, comienzan ha empeorar los síntomas y se genera un entorpecimiento de la evolución del cuadro” opinó.
Concordó en que si bien no se puede afirmar que el Roacnetan sea la causa del problema, ya que no existen estudios científicos al respecto, si se logra establecer una relación entre casos de depresión y uso del fármaco.
Héctor Fuenzalida, dermatólogo del Hospital Clínico de la Universidad de Chile, recomendó antes de recetarlo “tener la precaución, en pacientes con depresión o esas características, de recetar el Roacnetan. Yo dudaría en hacerlo o quizás controlaría la dosis del fármaco mucho más seguido, con un apoyo psiquiátrico detrás”.
El médico, que si bien prefirió relacionar la depresión a la baja autoestima que genera el acné, dijo que ante la duda es mejor tomar en cuenta las críticas que existen sobre el medicamento. Esto pese a que el fármaco “es un medicamento aprobado por la FDA y cumple todas las normas” concluyó el especialista.
3.) Mother of Suicidal Tampa Pilot Says She Will Sue
Maker of Accutane / Madre del joven que se suicido
intentara acción legal contra el fabricante de
Accutane (Roche).
Source:
http://www.injuryboard.com/
April 16, 2002
The mother of Charles Bishop, the 15-year-old pilot who
committed suicide on January 5 by flying a stolen Cessna
into a Tampa high rise, says Accutane is responsible for
her son's death. Julie Bishop recently told reporters in
an interview that because her son had shown no signs of
depression before the crash, "the only conclusion we
have been able to draw is the Accutane poisoned him."
Bishop said she plans to file suit against the drug's
maker.
A prescription for Accutane, made by Hoffman-La Roche,
was found in Bishop's home during a police search
shortly after the crash. Although an autopsy did not
find trace amounts of Accutane in Bishop's blood,
critics claim that the controversial medication can have
"depressive effects on the brain" weeks after a person
stops using it.

4.) Mother of 15-year-old Tampa suicide pilot blaming
acne drug for boy's death / 147 pacientes reporta FDA
con intentos de suicidio por Roaccutane desde su debut
en el mercado.
Source: http://www.injuryboard.com/
April 17, 2002, Wednesday
Associated Press
By VICKIE CHACHERE
The maker of the acne drug Accutane faces a $70
million lawsuit claiming the medicine caused severe
psychosis in a 15-year-old boy who crashed a stolen
plane into a Tampa high-rise. The lawsuit, filed
against Hoffmann-La Roche on Monday by the family of
Charles Bishop, blames Accutane for the boy's suicide.
Bishop slammed the small plane into the high rise on
Jan. 5 and left behind a note expressing sympathy for
Osama bin Laden and supporting the Sept. 11
attacks.
In an interview aired Tuesday by NBC's ``Today'' show,
the boy's mother, Julia Bishop, said her son had
showed no signs of depression.
``This child was a happy, well-balanced,
forward-thinking child who had a great deal to live
for,'' Bishop said. ``This was psychotic and the only
conclusion we have been able to draw is the Accutane
poisoned him.''
A Hoffmann-La Roche spokeswoman said the company was
unaware of the lawsuit and does not believe the drug
is dangerous.
Accutane carries a warning about suicide, but the
company points to statistics showing sufferers of
severe acne and teen-agers the main users of the drug
generally have higher suicide rates.
``We continue, as do the experts, to believe there is
no link,'' said company spokeswoman Carolyn Glynn.
The Food and Drug Administration says 147 people
taking Accutane either committed suicide or were
hospitalized for suicide attempts from 1982 to May
2000. An estimated 13 million patients have used
Accutane since its debut in 1982.
An autopsy found no trace of Accutane in Bishop's
blood, but attorneys for the family say so much blood
was lost in the crash that the test may not have been
useful.
Department of Diagnostic Sciences and Pathology,
Dental School, University of Maryland, Baltimore
21201-1586, USA. oral-path5@aol.com
BACKGROUND: The authors provide clinical findings in
five patients wearing oral jewelry to illustrate the
risks of experiencing periodontal injury associated
with body piercing involving intraoral and perioral
sites. They also present a literature review of other
adverse dental and medical consequences attributed to
oral piercing. CASE DESCRIPTIONS: Five young adult
patients with tongue and lip piercing sought dental
care. Each patient exhibited some degree of gingival
recession and mucogingival defects in proximity of
their oral jewelry. Three of these patients had
probing depths ranging from 5 to 8 millimeters in the
affected areas. CLINICAL IMPLICATIONS: Intraoral and
perioral jewelry may be associated with the
development of significant mucogingival deformities.
Because severe attachment loss can develop even when
gingival recession is minimal, it is critical that
patients with oral piercing routinely undergo
comprehensive periodontal assessment. The authors urge
clinicians to educate patients about the potential
risks regarding the practice of oral piercing.

5.) One year later, teen's mother, friend haunted by plane
crash death/Madre de joven que murió al estrellar avioneta
demanda al laboratorio Roche por 70 millones de dólares
!!!
Source: http://www.injuryboard.com/
Associated Press
January 06, 2003, Monday
One year after 15-year-old Charles Bishop crashed a stolen
airplane into a Tampa skyscraper, his mother and best friend
said they are still haunted by death and struggling with the
public attention it has drawn. Julia Bishop, who has filed a
$70 million lawsuit against the maker of the acne medication
Accutane that her son was taking before the crash, has made
few public statements since her son's death Jan. 5,
2002.
``This is a news story for the rest of the world,'' she told
the St. Petersburg Times in a story for Sunday's edition.
``But for me, he was my life and my son and it's extremely
difficult to have this thrown into my face all the
time.''
Bishop is limited in what she can talk about because of the
lawsuit, which contends Accutane caused the boy to develop
severe psychosis and led him to commit suicide. A trial is
set for March 2004 in U.S. District Court in Tampa.
Julia Bishop remembers her son, a high school freshman who
dreamed of becoming an Air Force Pilot, excitedly talking
about an upcoming trip to Australia and New Zealand as a
student ambassador.
Days later, he left a two-page note, expressing sympathy for
Osama bin Laden and supporting the Sept. 11, 2001, terrorist
attacks. He also claimed he had resisted recruiting attempts
by al-Qaida terrorists.
``When you see a pattern and then suddenly for somebody to
snap and think they are working for Osama bin Laden ... I'm
sure you've seen the note,'' Julia Bishop said. ``That's
really sick.''
Julia Bishop said she meets about once a month with her
son's best friend, Emerson Favreau, who says he still feels
guilty he didn't notice any suicide warning signs in his
friend.
Two days before the crash, Charles sent Favreau an e-mail
saying he was going to be on the news. Charles ended the
online conversation by saying: ``See you Monday.''
``That's what made me mad,'' Favreau said. ``Because he knew
he wasn't going to see me on Monday.''
Favreau has re-created his friend's final flight along
Tampa's skyline on an airplane computer simulator.
``I was trying to figure out maybe if there was something
going on,'' Favreau said. ``I still couldn't believe that he
did it on purpose.''
Emerson said he was finally convinced by a federal report in
November that said Bishop flew dangerously close to the
MacDill Air Force Base control tower before crashing into
the 42-story Bank of America Plaza in downtown Tampa.
``Before, there was nothing concrete that he had done it on
purpose,'' Favreau said. ``But you can't accidentally (buzz
a control tower) with a Cessna.''
Now a junior at East Lake High School, Favreau said students
don't talk much about Charles Bishop anymore, but that he is
still haunted by the suicide.
``It's just something that you can't get off your head,''
Favreau said.
![]()
6.) Constipation, polyuria, polydipsia, and edema associated with orlistat/ (XENICAL.) efectos adversos.
Ann Pharmacother. 2002 Jul-Aug;36(7-8):1168-70.
Packard KA, Wurdeman RL, Reyes AP.
Creighton Cardiac Center, Omaha, NE 68131-2044, USA. kelliot@cardiac.creighton.edu
OBJECTIVE: To report the occurrence of a novel group of adverse effects associated with initiation and rechallenge of orlistat. CASE SUMMARY: A 42-year-old white woman developed symptoms of constipation, polyuria, polydipsia, and increased lower-leg edema after 2 weeks of treatment with orlistat 120 mg 3 times daily. The drug was discontinued for 4 days and the symptoms resolved. On reinstitution of the orlistat treatment, the symptoms reappeared within 2 days. Thereafter, the medication was permanently discontinued. DISCUSSION: Common gastrointestinal adverse reactions associated with orlistat use include fecal urgency and abdominal pain and discomfort. Pedal edema has also been reported to occur, although less frequently. No reports were discovered documenting the occurrence of constipation, polydipsia, and polyuria associated with the use of orlistat. Despite careful consideration of other possible causes of these symptoms, the temporal association between initiation, discontinuation, and rechallenge of orlistat and the patient's symptoms suggest a medication-related adverse event. Based on the Naranjo probability scale, the likelihood that orlistat was the cause of this cluster of adverse effects is possible. CONCLUSIONS: It is important for the healthcare provider to be aware of these adverse effects to promptly evaluate and differentiate between possible causes of similar reactions.
7.) Orlistat e hipertension / Xenical e hipertension.
Source:
http://www.fonendo.com/
British Medical Journal 10/07/2000
El centro de Farmacovigilancia suizo ha reportado un caso de una paciente que hubo de retirar el fármaco por HTA.
Sin antecedentes de interés, comenzó el fármaco hasta llegar a una dosis de 120 mg/8 h. Comenzó a presentar mareo, edemas periféricos y cefalea pulsátil, constatándose unas cifras de TA de 190/100 mm Hg en tres ocasiones.
Tras retirar el fármaco, volvió a sus cifras normales. Tras reintroducirlo, volvió a presentar la misma sintomatología, por lo que se retiró definitivamente. La paciente se volvió a recuperar.
El fabricante había comunicado 13 casos previos de HTA, pero la información era incompleta. Aún no disponemos de recomendaciones al respecto, pero quizás sea necesario controlas las cifras de TA en los pacientes en tratamiento.
8.) INDIA: MEDICAMENTO VINCULADO A MUERTES DISPONIBLE
EN EL MERCADO (NIMESULIDE= AULIN, AINEX, SCAFLAN)
Source: http://www.boletinfarmacos.org/
Sanjay Kumar, BMJ, 2003; 326:70
El nimesulide, un anti-inflamatorio no esteroideo, se ha retirado de muchos mercados europeos pero sigue estando disponible en India. Se sabe que es un medicamento hepatotóxico y que ha provocado la muerte a varios niños. La India aprobó este medicamento en 1994 para uso en casos de inflamación musculoesquelética, sin embargo se utiliza frecuentemente como analgésico y antipirético.
Según el Dr Chandra Mohan Gulhati, editor del Boletín de Información sobre el Medicamento de India, el medicamento no está aprobado en EE.UU., Canadá, Australia, y partes de Europa; y Finlandia, España y Turquía lo retiraron del mercado el año pasado.
Después de que los medios de comunicación denunciaran el hecho, el responsable de controlar los medicamentos en India, Ashwini Kumar, dijo que el gobierno designaría a un comité de expertos para que estudiase el problema. El asistente del Sr. Kumar, Ram Teke, dijo a BMJ que el medicamento sigue estando disponible y que no había intención de reconsiderar su utilización o de retirarlo del mercado. El volumen de ventas de este medicamento es del orden de 1900 millones de rupíes (39,5 millones de dólares).
El nimeluside además de estar disponible como producto activo único, también esta presente en otros 30 medicamentos y en gotas para niños menores de un año. Todas estas combinaciones son ilegales porque no cuentan con el registro correspondiente.
El Dr. Gulhati dice que no hay un buen sistema para informar sobre reacciones adversas en India. Hay 12 medicamentos que se han retirado de los mercados globales, o que son de prescripción limitada, que están disponibles en la India, estos son: anagen, cerivastatina, droperidol, furazolidone, lynestrenol, nitrofurazona, fenformin, fenolftaleina, fenilbutazona, piperazina, y quiniodoclor.
Es más, cuando se decide retirar un medicamento del mercado, la implementación de esta decisión es muy deficiente, dijo el Dr Gulhati. Por ejemplo, en junio pasado se tomo la decisión de retirar los antialérgicos astemizole y terfenadine; la notificación no se hizo hasta octubre y en ella se indicaba que no se retirarían hasta agosto del 2003.
Según el Dr. Gulhati los medicamentos deberían retirarse del mercado tan pronto como se determina que son nocivos, y la única explicación plausible es que hay interés en proteger a la industria. Dice que las decisiones de la autoridad reguladora están afectadas por los intereses comerciales de los cabilderos de la industria y la corrupción; y que es una autoridad a la que no se le exige transparencia ni que justifique sus actividades.
Las acciones de los medios de comunicación han provocado que dos laboratorios recorten sus volúmenes de producción de nimesulide.
Ajay Kumar Handa, presidente de marketing para Centaur Pharmaceutical, dijo: " Ya no estamos produciendo jarabe de nimesulide." Y añadió “Por lo que yo sé no hay problemas con la administración de este medicamento a adultos.”
El grupo Social Jurist presentó un caso a la Corte Suprema de Delhi donde cuestiona la disponibilidad de nimesulide y de otros medicamentos que se han retirado de otros mercados pero que siguen estando disponibles en India.
Krezel W, Ghyselinck N, Samad TA, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863-867.
9.) WHAT IS DUTASTERIDE (AVODART™ AVOLVE™) /Nueva medicina para la caida del cabello !!!
Source: http://www.dutasteridedirect.com/
Dutasteride (Avodart, Avolve) - a new, emerging treatment for hair loss DutasterideDIRECT.com is your online source for news and information on the latest treatments for hair loss. This FAQ has been compiled from a number of sources to give an overview of the use and effects of Dutasteride.
What is Dutasteride (Avodart, Avolve)?
Avolve is the European brand name for Dutasteride, an oral
medicine made by GlaxoSmithKline for treating symptomatic
benign prostatic hyperplasia (BPH) in men.
The active compound in Avolve, Dutasteride, has the added
benefit of treating genetic male pattern hair loss on the
vertex (top of the head) and the mid-scalp area.
Dutasteride is chemically similar to Finasteride, the
active compound in Propecia.
Avolve is also known by its US brand name, Avodart. The
medicine has been approved by the US Food & Drug
Administration, and in Europe, for treating BPH. Avolve is
supplied in 0.5mg soft gelatin capsules.
How does Evolve work?
Researchers have discovered that men who suffer from
benign prostatic hyperplasia (BPH), or from male pattern
hair loss, have increased levels of the hormone
dihydrotestosterone (DHT).
DHT is produced from testosterone by an enzyme called
5-alpha-reductase. Biochemical analysis reveals higher
levels of 5-alpha-reductase in the bloodstream of men with
BPH, and in the scalps of men with hair loss; and less of
this enzyme in men with no BPH or no hair loss.
Avolve inhibits 5-alpha-reductase, blocking the formation
of DHT. This interrupts a key trigger element in both BPH
and development of male pattern hair loss.
What is dihydrotesterone (DHT)?
DHT is one of several male hormones in the body. DHT is
responsible for the development of the external genitals
in the male foetus. However, in adult males DHT appears to
cause:
male pattern hair loss
prostate enlargement
shortening of the growing phase of hair
progressive miniaturisation of hair follicles
decreasing number of visible hairs
acne
How is Avolve different from Propecia?
Both medicines work in a similar way. However, Avolve
(Dutasteride) inhibits the activities of two types of
5-alpha-reductase enzymes. In contrast, Propecia
(Finasteride) only inhibits one type. Avolve has been
shown to decreases levels of DHT by 90% after only two
weeks, making it a more powerful and faster-acting weapon
against BPH and hair loss than Finasteride.
What studies and trials have been done concerning
Avolve?
A total of 2951 men with moderate to severe BPH were
treated with 0.5 mg Avolve (dutasteride) daily. The study
found that acute urinary retention was reduced by 57%, and
the risk of benign prostatic hyperplasia-related surgical
intervention was cut by 48% compared with placebo. The
drug was shown to be well tolerated.
GlaxoSmithKline also completed Phase II trials for FDA
approval of Avolve for treating hair loss. After six month
of treatment, the hair counts measured in a 1 inch
diameter circle increased by an average 96 hairs with
0.5mg Avolve daily, compared to an average 72 hairs with
5mg Propecia (Finasteride) daily.
So these initial trials show that Avolve is around
30% more effective than Propecia in promoting hair
regrowth. However, please note that Avolve has only been
specifically approved for treating BPH. It has not yet
been approved specifically for treating hair loss.
When will Avolve be approved for treating hair loss?
In November 2002 GlaxoSmithKline cancelled its planned
Phase 3 trials for Avolve for treating hair loss. The
company has not publicly given a reason for this. Industry
sources speculate that the trials were stopped because the
maker thinks Avolve will be perceived as too similar to
Propecia in consumers' minds, and may not generate
sufficient return on investment to justify the cost of the
approvals process as a treatment for hair loss.
However, Avolve is approved by the US FDA and by European
bodies for the treatment of BPH, and so has passed all
relevant safety standards.
Will Avolve help hair regrowth for all men?
As with Propecia, Avolve increases the number of scalp
hairs, helping to fill-in thin areas of the scalp.
Although results will vary, generally men will not re-grow
all of the hair they have lost. Male pattern hair loss
occurs gradually over time, but Avolve can significantly
reduce or delay hair loss. Note that Evolve is not yet
approved for treating hair loss: its effects are a
side-effect of its actions against BPH.
Is Evolve safe?
Clinical trials showed that it was generally well
tolerated. Most side effects were mild or moderate and
generally went away while on treatment in both the Avolve
and placebo groups.
Drug-related side effects during the first six months were
as follows:
impotence (4.7% vs. 1.7% for placebo)
decreased libido (3% vs. 1.4%)
breast tenderness and breast enlargement (gynecomastia;
0.5% vs. 0.2%)
ejaculation disorders (1.4% vs. 0.5%).
Avolve should not be used in women and children. Women who
are pregnant or may become pregnant should not handle this
medicine because of possibility of absorption and
subsequent potential risk to a male foetus.
Men treated with Avolve should not donate blood until at
least six months after their final dose to prevent the
medicine going to a pregnant woman through a blood
transfusion.
Men with liver disease should talk to their doctor before
taking Avolve.
![]()
10.) AVODART™ / DUTASTERIDE/ The Biggest News In Hair Loss Treatment Since Propecia.
Source: http://www.hairsite2.com/
FDA clearance for Avodart or dutasteride was granted to
GlaxoSmithKline on October 9, 2002. This is the biggest
news in hair loss treatment since Propecia. This page is
updated regularly based on discussions in DUTASTERIDE
FORUM. If you want the latest discussions, debates and
developments about Avodart or Dutasteride, visit the
DUTASTERIDE FORUM. But if you just want to read about
forum summaries and important issues about Avodart or
Dutasteride on an ongoing basis, this page will be very
helpful. Most information here are courtesy of posters in
the Dutasteride Forum.
WHAT IS AVODART OR DUTASTERIDE?
After 30 Dutasteride forums in HairSite and endless
speculation for almost two years, Dutasteride is finally a
reality under the brand name Avodart™ !
The FDA approval for NDA#21-319/s-001 was given on October
9, 2002 and the label was posted on the same day.
This is the biggest news in hair loss treatment since
FDA's clearance for Propecia in December 1997. The
interesting thing is that Avodart is NOT a hair loss drug
!!
Avodart™ (Dutasteride) is approved for the treatment of
Benign Prostatic Hyperplasia (BPH). Not to be mistakened
with prostate cancer, BPH is a non-cancerous enlargement
of the prostate glands. It is fairly common among older
men aged 50 or above. BPH usually manifest in a variety of
lower urinary tract symptoms (LUTS) such as urinary
frequency, urgency, nighttime urination, decrease in
urinary stream, incomplete emptying and incontinence
etc.
Avodart is a synthetic 4-azasteroid compound. It is the
first FDA approved medication that can inhibit both types
of 5-alpha reductase (5AR), an enzyme that is commonly
believed to responsible for the conversion of testosterone
to 5 alpha-dihydrotestosterone (DHT). It is widely
suggested that DHT is the main androgen responsible for
prostate growth as well as male pattern baldness.
ACTIVE INGREDIENTS
Avodart standard dosage is 0.5mg of the active ingredient
dutasteride per soft gelatin capsule. The capsule is
compounded by dissolving 0.5mg of dutaseride in a
combination of mono-di-glycerides caprylic/capric acid and
butylated hydroxytoluene. Other ingredients include
BSE-free bovine gelatin, glycerin and yellow ferric oxide.
TOPICAL DUTASTERIDE
If you are able to obtain Dutasteride in its raw form, you
might even be able to compound your own Dutasteride
topical solution. It was reported in Avodart's prescribing
information that the medication is absorbed through the
skin as well. Dutasteride in its raw form is a
white-yellowish powder. It is insoluble in water but can
easily be dissolved in ethanol (44mg/mL), methanol (64
mg/mL) and polyethylene glycol 400 (3mg/mL). However,
please note that effectiveness of Dutasteride or Avodart
as a hair loss treatment has not been clinically
established.
You may want to follow this thread for more thorough
discussion about topical dutasteride.
DUTASTERIDE vs FINASTERIDE
Unlike Finasterdie (Propecia or Proscar) which only
inhibits Type II 5AR, Dutasteride functions as a dual 5AR
inhibitors and have been proven to be more effective as an
treatment for BPH and possibly pattern hair loss or
baldness. According to GSK, Dutasteride has shown to
exhibit significantly less inter-individual variation in
the level of DHT suppression compared to Finasteride.
A comparison between Avodart (Dutasteride) and Proscar
(Finasteride 5 mg) can be found in Dutasteride forum.
STANDARD DOSE
Scientists have evaluated the effectiveness of Dutasteride
at different daily dosage, ranging from 0.01 mg, 0.05 mg,
0.5 mg, 2.5 mg, and 5.0 mg per day. It was found that the
highest suppression of DHT was achieved with 2.5 mg or 5.0
mg per day. At a daily dosage of 2.5 mg or 5.0 mg,
Dutasteride suppresses close to 100% of the DHT whereas
5.0 mg daily dosage of Finasteride only suppresses close
to 70% of the DHT. According to information provided by
GSK, the level of DHT suppression does not seem to
different significantly between 2.5 mg and 5.0 mg. And at
a daily dose of 0.5mg, DHT inhibiton is close to 90%.
Please also read below OPTIMAL DOSE FOR HAIR LOSS.
MAXIMUM EFFECT
According to Avodart or dutasteride's prescribing
information, the maximum effect of dutasteride can be
observed within 1 to 2 weeks. "After 1 and 2 weeks of
daily dosing with dutasteride 0.5mg, median serum DHT
concentrations were reduced by 85% and 90% respectively."
It was further reported that test patients treated with a
daily dosage of 0.5mg dutasteride for 2 years, the median
decrease in serum DHT concentration was over 92% for both
year 1 and year 2 of the 2-year trial.
SIDE EFFECTS, WARNINGS, CONCERNS
1) Women or children should not use Avodart™ or
dutasteride.
2) Women who are pregnant or may be pregnant should not
handle Avodart™ for fear of potential risks to a male
fetus.
3) No measurable bone mineral density level change was
observed in test patients after a 52 weeks trial.
4) Men using Avodart™ or dutasteride should not donate
blood until at least 6 months after the medication was
last used for fear of possible administration of
dutasteride tainted blood to pregnant female transfusion
patient.
5) Men with liver problems should not use Avodart™.
6) Patients' ejaculate volume might decrease as a result
of the medication. A small number of the test patients
experienced impotence and a reduction in libido.
7) No clinically significant change in hormone levels were
observed in test patients.
8) No clinically significant changes in sperm
concentration, sperm motility or sperm morphology although
some subjects reported reduction in ejaculate volume.
9) A 2-year statistical summary showed that sexual adverse
events such as impotence, decreased libido, and
ejaculation disorder decreased with the duration of the
treatment. Fewer incidences of adverse sexual events were
reported over time.
OPTIMAL DOSAGE FOR HAIR LOSS
For the treatment of BPH, the official recommendation from
GSK is to take 1 x 0.5mg capsule orally every day with or
without food.
However, it appears that for treating hair loss, a daily
dose of 2.5mg daily seems to generate the best results.
See Dutasteride Results.
Also, please follow this thread in Dutasteride Forum which
discussed the effect of Avodart on serum DHT vs scalp DHT.
0.5mg Dutasteride inhibits ~ 92% serum DHT and 55% scalp
DHT
2.5mg Dutasteride inhibits ~ 95% serum DHT and 82% scalp
DHT
DarkMage and Bryan in the Dutasteride forum have also
provided very valuable information about the optimal daily
dose for dutasteride. You can find their charts and
analysis at "Optimal Daily Dose".
WITH OR WITHOUT FOOD
Please read Bryan's post and his interpretation about
whether Avodart should be taken with or without food.
GENUINE AVODART™
Please note that genuine Avodart™ are oblong, opaque, dull
yellow, gelatin capsules with the word "GX CE2" clearly
printed in red on one side. Avodart™ is packaged in
bottles of 100 (NDC 0173-0712-00) with child resistant
closures. You may also find genuine Avodart™ in blister
packs of 70 capsules (NDC 0173-0712-01).
STORAGE
15-30 degrees Celsius or 59 - 86 degrees F.
HALF-LIFE
Discussions here about half-life are mostly courtesy of
Bryan in Dutasteride Forum.
Half-life refers to the time (usually in hours) required
for one-half the dose of a drug to be excreted from the
body. The following half-life information about Avodart™
or dutasteride is found in the prescribing information:
"The terminal half-life of dutasteride is approximately 5
weeks at steady state. The average steady-state serum
dutasteride concentration was 40 ng/mL following 0.5
mg/day for 1 year. Following daily dosing, dutasteride
serum concentrations achieve 65% of steady-state
concentration after 1 month and approximately 90% after 3
months. Due to the long half-life of dutasteride, serum
concentrations remain detectable (greater than 0.1 ng/mL)
for up to 4 to 6 months after discontinuation of
treatment. "
Avodart or Dutasteride has relatively much longer
half-life than Propecia. However, it does not necessarily
mean that you can reap substantial cost savings because in
the long run, you have to sustain the same amount of
medication in your body in order to see results. You might
be able to "defer" the money you spent on Avodart, but not
cutting the overall amount you spend. The real
significance of long half-life is that it allows for very
flexible dosing schemes. You can take a small amount daily
or a larger dose less frequently. But the key is to
average the same amount. For example, if your goal is to
average 0.5mg in your body, then you can take 0.5mg daily
or 3.5mg weekly.
Note that while Avodart or Dutasteride has a long
half-life, it does not necessarily mean that the
medication will simply stay fixed at the highest "peak"
level all the time.
DR. LEE'S RESPONSE RE: PRESCRIBING AVODART FOR HAIR
LOSS
October 10, 2002 post - This is from HairSite's
Dutasteride forum. Note that we have not verified if the
email is indeed from Dr. Richard Lee at Regrowth.
AVAILABILITY & PRICE
Please read the anchored topic in the Avodart forum,
availability and pricing is contantly updated there.
GETTING A SCRIPT
Someone has come across www.medicalwellnesscenter.com
which will write us a script for about $50. Many have
already tried their service. The beauty of this service is
that we are NOT obligated to purchase Avodart from any
pharmacy. We can choose to fill the script at ANY pharmacy
of our choice. This site is legitimate and if you want to
read about posters' comments about this website's service,
please go to Avodart forum 35. Non U.S residents can take
advantage of their service as well.
AVODART / DUTASTERIDE FORUM
--------------------------------------------------------------------------------
AVODART™ IS INTENDED FOR THE TREATMENT OF BENIGN PROSTATE
ENLARGEMENT. PLEASE CHECK WITH YOUR DOCTOR IF HE WILL
PRESCRIBE AVODART™ AS A HAIR LOSS TREATMENT.
AVODART™ is a registered trademark of GlaxoSmithKline.
11.) Synopsis of Hereditary Poikilodermatous syndromes.
Khalid Al Aboud,M.D
Khalid Al Hawsawi,M.D
Dermatology division,Department of Medicine,King Faisal Hospital,Taif,Saudi Arabia
Address of corressopndence :Khalid Al Aboud,M.D,P.O Box 5440
Makkah,Saudi Arabia,Tel 966 2 7382444,Fax 966 2 7384719,e-mail amoa65@hotmail.com
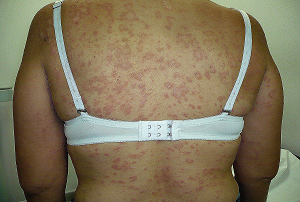
Aging of the skin is a physiological process.However,there are a groups of diseases that cause aging of the skin.One of these group is hereditary poikilodermatous syndromes.It is characterixed by affection of the skin by ,pigmentary changes(both hypo and hyperpigmentation),atrophy and telangiectasias(fig .1)
This group include:
1 Rothmund- Thomson Syndrome.
2 Dyskeratosis Congenita.
3 Xeroderma Pigmentosum.
4 Kindler Syndrome.
5 Epidermolysis Bullosa Simplex Variants.
Table I provide a synopsis for the main diseases in this group.
TableI:Synopsis of the main hereditary poikilodermatous syndromes.
|
|
|
|
|
|
|
|
|
|
|
|
|
POIKILODERMA |
|
|
ABNORMAL DENTITION |
|
|
|
|
|
|
|
|
Oral CA |
|
|
|
|
|
|
|
+ |
ANORECTAL & URETHRAL STENOSIS |
DISORDERS, NEOPLASIA. |
|
|
|
BIRTH:
|
|
|
|
|
|
|
WEARY SYNDROME WEAR (JET AL 1971) |
|
|
|
|
- |
|
- |
Figure 1:poikilodermatous skin changes in a patient with
Kindler syndrome.
======================================================================
DATA-MÉDICOS/ DERMAGIC JOURNAL/ AUGUST 2.003-2024/ DR. JOSE LAPENTA
R.
=====================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.0024
Tlf: 0414-2976087 - 04127766810



















.png)