FINASTERIDE 5 Mg VS FINASTERIDE 1 Mg EN LA ALOPECIA ANDROGÉNICA, ACTUALIZACIÓN
Hola amigos de la red DERMAGIC, de nuevo con ustedes con un tema bien caliente: FINASTERIDE 5 VS FINASTERIDE 1 EN ALOPECIA ANDROGÉNICA.
El primer trabajo que encontré sobre esta droga data del año 1.993 y otros más de 1.994 donde se hablaba del FINASTERIDE COMO UNA DROGA promisoria en algunas PATOLOGÍAS COMO Acné, Hirsutismo, Cáncer de próstata e Hiperplasia Prostática Benigna (HPB).
PERO NUNCA se habló del FINASTERIDE COMO una droga ÚTIL en la Alopecia Androgénica hasta los años 1.998, cuando el laboratorio Merck S. and Dhome decide mercadear LA PROPECIA (Finasteride 1 Mg) para su uso en la caída del cabello de origen hormonal.
Por supuesto ya se había comprobado su efecto ANTI ANDROGÉNICO.
Pero la pregunta es la siguiente ???. Porque el Laboratorio M.S.D le quita 4 mgrs al PROSCAR Finasteride 5 mgrs) para comercializarlo como un PRODUCTO "NUEVO", con el nombre de PROPECIA de 1 Mg ???, si ya para los años 1.995 se había comprobado su eficacia en la Hiperplasia Prostática Benigna (HPB), y caída del cabello de origen androgénico ?
EXISTEN estudios donde a pacientes se les dio FINASTERIDE 5 MGRS DÍA POR 2 AÑOS SIN EFECTOS COLATERALES, MÁS AÚN,, en el primer reporte de 1.993 se DEMOSTRÓ que dosis diarias de 80 MGRS /día de finasteride por 3 meses NO OCASIONABAN EFECTOS SECUNDARIOS, posteriormente si se reportaron efectos secundarios.
Por otra parte se ha demostrado que dosis diarias de PROPECIA (Finasteride 1 Mg) han provocado GINECOMASTIA y ADENOMAS, los cuales fueron reversibles con la suspensión del tratamiento. Pero solo hay unos 3 o 4 casos reportados, de una cohorte de miles pacientes que han tomado FINASTERIDE.
Desde que se conoció en el mundo científico QUE EL FINASTERIDE producía crecimiento del cabello, comenzó a usarse el PROSCAR (FINASTERIDE 5 mgrs), porque la PROPECIA (Finasteride 1 Mg) no había salido al mercado. La dosis: 10 Mg SEMANALES en 2 dosis, martes y jueves mostraron BUEN RESULTADO.
Una vez salida la PROPECIA al MERCADO (Finasteride 1 Mg), el laboratorio comenzó una AGRESIVA CAMPAÑA descalificando este esquema terapéutico, (5 mgrs dos veces semanal), diciendo que la dosis tenia que ser un 1 mgr día por 7 días a la semana.
Pero la VERDAD es que FINASTERIDE 5 Mg 2 VECES SEMANAL es tan bueno o superior al FINASTERIDE 1 Mg DIARIO, y el costo es muchísimo menor, más seguro, menos riesgos. Una caja de 30 tab dura 15 semanas, (3 meses y 3 semanas).
Entonces podríamos pensar LÓGICAMENTE, que el laboratorio MERCK.S. DOHME lo que hizo fue mercadear un "viejo" y buen producto, reduciendo la dosis PARA QUE LO TOMÁRAMOS TODOS LOS DÍAS, en fin una mejor manera de "EXPLOTAR" la droga.
Yo me fui por el lado científico y comencé a probar el producto PROSCAR (Finasteride 5 mgrs) en pacientes con Alopecia Androgénica con el esquema antes dicho: 5 mgrs 2 veces semanal y al 4to mes observe mejoría notable de los pacientes, vean las fotos del attach.
He notado los siguientes efectos con el uso del FINASTERIDE: Rápida repoblación del cabello áreas frontal y occipital. MUCHO MÁS RÁPIDA que las fotos que nos muestra el LABORATORIO con 1 y 2 años de tratamiento con FINASTERIDE 1 Mg diario. efectos adversos: NINGUNO, solo una leve disminución de la cantidad del volumen del semen, de resto TODO NORMAL..
CONCLUSIONES:
1.) EL FINASTERIDE ES UN producto maravilloso, en sus dos presentaciones (5 Mg y 1 Mg).
2.) FINASTERIDE 5 Mg 2 veces semanal es MEJOR o Igual QUE FINASTERIDE 1 Mg DIARIO.
3.) EL LABORATORIO MERCK.S.D LANZÓ la PROPECIA con fines COMERCIALES Y DE MERCADEO.
4.) FINASTERIDE MEJORA LA HIPERPLASIA PROSTÁTICA BENIGNA (HPB), Y PRODUCE mejoría notable en la ALOPECIA ANDROGÉNICA.. !!!
No duden que por allí vendrá el FINASTERIDE TÓPICO, ya se está hablando de ello... (referencia 33).
Para el año 2024 fue FINALMENTE aprobado el FINASTERIDE TÓPICO, el cual se presenta solo o en combinación con el popular MINOXIDIL, el cual también es utilizado en la caída del cabello.
En este enlace encontrarás la actualización del FINASTERIDE 5 Mg VS FINASTERIDE 1 Mg EN LA ALOPECIA ANDROGÉNICA (2025), con más información y referencias sobre su uso, y también sus efectos secundarios.
Saludos a todos !!!
Dr. José Lapenta R.,,,
EDITORIAL ENGLISH:
Hello friends of the DERMAGIC network, back with a very hot topic: FINASTERIDE 5 VS FINASTERIDE 1 IN ANDROGENIC ALOPECIA.
The first article I found on this drug dates back to 1993, and others from 1994 onward. FINASTERIDE was discussed as a promising drug for certain conditions such as acne, hirsutism, prostate cancer, and benign prostatic hyperplasia (BPH).
But FINASTERIDE was never discussed as a useful drug for Androgenetic Alopecia until 1998, when the Merck S. and Dhome laboratory decided to market PROPECIA (Finasteride 1 mg) for use in hormonal hair loss.
Of course, its ANTI-ANDROGENIC effect had already been proven.
But the question is this: Why does MSD Laboratory remove 4 mg from PROSCAR (Finasteride 5 mg) to market it as a "NEW" product, under the name PROPECIA 1 mg? Even though its effectiveness in Benign Prostatic Hyperplasia (BPH), and androgenic hair loss had already been proven in 1995?.
There are studies where patients were given FINASTERIDE 5 mg daily for 2 years without side effects. Furthermore, in the first report from 1993, it was demonstrated that daily doses of 80 mg/day of FINASTERIDE for 3 months did not cause side effects. Later, side effects were reported.
On the other hand, it has been shown that daily doses of PROPECIA (Finasteride 1 mg) have caused GYNECOMASTIA and ADENOMAS, which were reversible upon discontinuation of treatment. However, only 3 or 4 cases have been reported from a cohort of thousands of patients who have taken finasteride.
Since it became known in the scientific world that FINASTERIDE cause hair growth, PROSCAR (Finasteride 5 mg) began to be used, because PROPECIA (Finasteride 1 mg) had not yet been marketed. The dose: 10 mg weekly in 2 doses, Tuesdays and Thursdays, showed good results.
Once PROPECIA (finasteride 1 mg) was released, the company began an aggressive campaign discrediting this therapeutic regimen (5 mg twice weekly), stating that the dose should be 1 mg daily, 7 days a week.
But the TRUTH is that FINASTERIDE 5 mg twice a week is as good as or better than FINASTERIDE 1 mg daily, and the cost is much lower, safer, and there are fewer risks. A box of 30 tablets lasts 15 weeks (3 months and 3 weeks).
So we could logically think that what the MERCK.S. DOHME laboratory did was market an "old" and good product, reducing the dose SO THAT WE WOULD TAKE IT EVERY DAY, in short, a better way to "EXPLOIT" the drug.
I went for the scientific route and began testing the product PROSCAR (Finasteride 5 mg) in patients with Androgenetic Alopecia with the aforementioned regime: 5 mg twice a week. After the 4th month, I observed a notable improvement in the patients. See the attached photos.
I have noticed the following effects with the use of FINASTERIDE: Rapid regrowth of hair in the frontal and occipital areas. MUCH FASTER than the photos shown by the laboratory after 1 and 2 years of treatment with FINASTERIDE 1 mg daily. Adverse effects: NONE, only a slight decrease in semen volume; otherwise, everything is normal.
CONCLUSIONS:
1.) FINASTERIDE IS A wonderful product, in both forms (5 mg and 1 mg).
2.) FINASTERIDE 5 mg twice weekly is BETTER or EQUAL TO FINASTERIDE 1 mg DAILY.
3.) MERCK S.D. LAUNCHED PROPECIA FOR COMMERCIAL AND MARKETING PURPOSES.
4.) FINASTERIDE IMPROVES BENIGN PROSTATIC HYPERPLASIA (BPH) AND PRODUCES NOTABLE IMPROVEMENT IN ANDROGENIC ALOPECIA...!!!
Don't doubt that TOPICAL FINASTERIDE is coming, it's already being talked about... (reference 33).
TOPICAL FINASTERIDE was FINALLY approved in 2024. It is available alone or in combination with the popular MINOXIDIL, which is also used for hair loss.
At this link, you will find the update on FINASTERIDE 5 mg VS FINASTERIDE 1 mg IN ANDROGENCI ALOPECIA (2025), with more information and references on its use, as well as its side effects.
Greetings to all!!!
Dr. José Lapenta R.
===============================================================
REFERENCIAS BIBLIOGRÁFICAS / BIBLIOGRAPHICAL REFERENCES
================================================================
1.) Five-year follow-up of patients with benign prostatic hyperplasia treated with finasteride.
2.) Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomized double-blind controlled trial.
3.) The effect of finasteride on prostate volume, urinary flow rate and symptom score in men with benign prostatic hyperplasia.
4.) [5-alpha-reductase inhibitors].
5.) Benign prostatic hyperplasia.
6.) The potential for hormonal prevention trials.
7.) 5 alpha-reductase inhibition by finasteride (Proscar) in epithelium and stroma of human benign prostatic hyperplasia.
8.) Pharmacological treatment of benign prostatic hyperplasia with finasteride: a clinical review.
9.) Medical therapy for benign prostatic hyperplasia: A review of the literature.
10.) Pretreatment with finasteride decreases perioperative bleeding associated with transurethral resection of the prostate.
11.) Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. Characterization of patients and ultimate outcomes.The PLESS Study Group.
12.) Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia.
13.) Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men.
14.) Management of androgenetic alopecia.
15.) Finasteride in the treatment of men with frontal male pattern hair loss.
16.) Androgenetic alopecia in men: the scale of the problem and prospects for treatment.
17.) [Androgenetic alopecia, hirsutism and hypertrichosis].
18.) Medical treatments for balding in men.
19.) Understanding and managing common baldness.
20.) Finasteride: a review of its use in male pattern hair loss.
21.) Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group.
22.) Genetic analysis of male pattern baldness and the 5alpha-reductase genes.
23.) Effect of finasteride on human testicular steroidogenesis.
24.) [Finasteride: a new drug for the treatment of male hirsutism and androgenetic alopecia]?
25.) The 5 alpha-reductase system and its inhibitors. Recent development and its perspective in treating androgen-dependent skin disorders.
26.) Finasteride: a clinical review.
27.) The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness.
28.) Finasteride: the first 5 alpha-reductase inhibitor.
29.) Cytologic atypia in a 53-year-old man with finasteride-induced gynecomastia.
30.) Reversible painful gynaecomastia induced by low dose finasteride (1 mg/day).
31.) Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts
in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men and postmenopausal women.
32.) Improvement in androgenetic alopecia in 53-76-year-old men using oral finasteride.
33.) New topical antiandrogenic formulations can stimulate hair growth in human bald scalp grafted onto mice.
34.) Current management of androgenetic alopecia in men.
35.) Immunohistochemical localization of types 1 and 2 5alpha-reductase in human scalp.
36.) The psychosocial consequences of androgenetic alopecia: a review of the research literature.
37.) Clinical dose ranging studies with finasteride, a type 2 5alpha-reductase inhibitor, in men with male pattern hair loss.
38.) The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia.
=============================================================
=============================================================
1.) Five-year follow-up of patients with benign prostatic hyperplasia treated with
finasteride.
=============================================================
Eur Urol 1995;27(4):267-73
Geller J
Mercy Hospital and Medical Center, San Diego, CA 92103-2180, USA.
In 18 of 55 original patients who completed 5 years of treatment with finasteride, significant reductions in prostate size were noted at 1 year and sustained thereafter. Symptom scores in these same patients were significantly improved or stable over the 5 years while maximal urinary flow rates were unchanged. Data from 15 of 18 other patients who dropped out of the study before 5 years showed changes in prostate size, symptom score and flow rates that were similar to those noted in patients treated for 5 years. No side effects were noted in this study except for sexual dysfunction, which occurred in less than 5% of the patients. With few exceptions, finasteride appears to arrest the process of BPH over a 5 year period as indicated by sustained reductions in prostate size accompanied by either symptomatic improvement or stability in all other patients.
=============================================================
2.) Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomized
double-blind controlled trial.
=============================================================
J Formos Med Assoc 1995 Jan-Feb;94(1-2):37-41
Yu HJ, Chiu TY, Lai MK
Department of Urology, National Taiwan University Hospital, Taipei, R.O.C.
The clinical effects of finasteride, a 5 alpha-reductase inhibitor, in patients with benign prostatic hyperplasia (BPH) were evaluated in a double-blind, placebo-controlled study. Forty-six patients with symptomatic BPH were randomly assigned to 2 groups, the finasteride group and the placebo group. The finasteride group received 5 mg of finasteride daily for 6 months. Prostate volume, urinary flow, urinary symptoms, serum prostate-specific antigen (PSA) and adverse events were determined before and after treatment. After 6 months of treatment the patients treated with 5 mg of finasteride per day had a 30% decrease in their total urinary symptom score, a 14% decrease in prostate volume and a 0.9 ng/dL decrease of PSA. Their maximal urinary flow rate increased by 1.42 mL per second and the mean urinary flow rate increased by 0.64 mL per second. The patients given placebo showed no significant changes in their prostate volume, serum PSA and maximal and mean urinary flow rate. However, the symptom scores in the placebo group also decreased significantly. When compared with the placebo group, those in the finasteride group had significantly lower prostate volume, serum PSA, maximal urinary flow rate and urinary symptoms, but not mean urinary flow rate. The frequency of adverse events was low in both the finasteride and placebo groups. These results show that finasteride may be an effective and safe alternative for the treatment of patients with BPH.
=============================================================
3.) The effect of finasteride on prostate volume, urinary flow rate and symptom score in
men with benign prostatic hyperplasia.
=============================================================
Aust N Z J Surg 1995 Jan;65(1):35-9
Nacey JN, Meffan PJ, Delahunt B
Department of Surgery, Wellington School of Medicine, New Zealand.
This study was designed to determine the efficacy of the 5 alpha-reductase inhibitor finasteride (Proscar, MK-906) in men with reduced urinary flow rates and symptoms of urinary outflow obstruction secondary to benign prostatic hyperplasia. Forty-five men were randomized to one of three groups receiving either placebo, 1 mg/day or 5 mg/day finasteride for the first 12 months of the study period. At the end of this period all men received 5 mg/day finasteride for a further 2 years. Efficacy was determined by measurement of prostate volume, maximum urinary flow rate, and symptom score using a modified Boyarsky assessment. Prostate volume reduced by 20 and 27%, respectively, for those on 1 and 5 mg after the first year. At 3 years the volume had reduced by 43%. This reduction in prostate volume was associated with an improvement in maximum urinary flow rate by 50% (1 mg), and 35% (5 mg) at 1 year, and 36% at 3 years. The total, obstructive and non-obstructive symptom scores decreased (improved) for patients on 1 and 5 mg finasteride, with the total score reducing by 33% from baseline at year 3. The results demonstrate that finasteride causes a modest but significant clinical improvement in men with urinary outflow obstruction secondary to benign prostatic
hyperplasia.
=============================================================
4.) [5-alpha-reductase inhibitors].
=============================================================
Acta Urol Belg 1994 Dec;62(4):23-31
De Jaegher K, Kozyreff P, Claes H
A reflection is made, on the one hand, on the lack of correlation between the intensity of micturition problems and the volume of the prostate and, on the other hand, on the different therapeutic approaches of irritative or obstructive voiding problems, and finally on the insufficiently convincing activity of Finasteride.
=============================================================
5.) Benign prostatic hyperplasia.
=============================================================
Endocrinol Metab Clin North Am 1994 Dec;23(4):795-807
Jonler M, Riehmann M, Brinkmann R, Bruskewitz RC
Division of Urology, University of Wisconsin, Madison.
Benign prostatic hyperplasia (BPH) is the most common cause of bladder outlet obstruction and voiding symptoms in elderly men. The pathogenesis is not fully determined but a combination of androgens and age are needed for development of BPH. Symptoms of BPH are divided into obstructive and irritative symptoms but large interpersonal variability is found and no specific BPH symptom exists. Treatment modalities include surgery (TURP, TUIP, open prostatectomy, laser ablation, balloon dilatation, hyperthermia and thermotherapy, and urethral stents) and medical therapy. TURP is the gold standard treatment and TUIP is a safe and effective alternative to TURP in patients with smaller prostates. Laser ablation, hyperthermia and thermotherapy, and urethral stents are at the present time under investigation. Balloon dilatation is FDA-approved but not often used because of low efficacy and poor long-term results. Medical treatment includes alpha-blocker or finasteride treatment and is indicated in patients with moderate to severe symptoms of BPH without a strong indication for surgery.
=============================================================
6.) The potential for hormonal prevention trials.
=============================================================
Cancer 1994 Nov 1;74(9 Suppl):2726-33
Ford LG, Brawley OW, Perlman JA, Nayfield SG, Johnson KA, Kramer BS
Detection and Community Oncology Program, National Cancer Institute, Bethesda,
Maryland 20892.
Breast and prostate cancer are significant causes of morbidity and mortality and are very similar in etiology, epidemiology, and modalities of treatment. Investigational strategies in the prevention of these malignancies also have strong parallels. The National Cancer Institute is sponsoring several large scale clinical trials involving hormonal manipulation and cancer prevention. In the Breast Cancer Prevention Trial, 16,000 women at high risk for breast cancer are being randomized to receive the antiestrogen agent tamoxifen or placebo for 5 years in an effort to determine if breast cancer development can be inhibited. In a similar trial, the Prostate Cancer Prevention Trial, 18,000 men older than 55 years of age will be randomized to receive finasteride, a 5-alpha-reductase inhibitor, or placebo to determine if inhibition of dihydrotestosterone synthesis in the prostate over a prolonged period will lead to a decreased incidence of prostate cancer. Both clinical trials offer the possibility of demonstrating that a hormonal intervention can decrease an individual's risk of developing breast or prostate cancer. They also have the potential of providing critical information about cancer risk, etiology, screening, and genetics, as well as quantifying the risks and benefits of specific preventive interventions.
=============================================================
7.) 5 alpha-reductase inhibition by finasteride (Proscar) in epithelium and stroma of human
benign prostatic hyperplasia.
=============================================================
Steroids 1994 Nov;59(11):616-20
Weisser H, Tunn S, Debus M, Krieg M
Institute of Clinical Chemistry and Laboratory Medicine, University Clinic Bergmannsheil,
Bochum, Germany.
Finasteride is a specific 5 alpha-reductase inhibitor that has been shown to reduce the size of human benign prostatic hyperplasia (BPH) by inhibiting the intraprostatic conversion of testosterone to 5 alpha-dihydrotestosterone. The aim of the present in vitro study was to describe in more detail the inhibitory effect of finasteride on 5 alpha-reductase in epithelium and stroma of human BPH. 5 alpha-Reductase activity in epithelium and stroma was inhibited dose-dependently by finasteride. The mean IC50 (50% inhibitory concentration) values, determined in the presence of various testosterone concentrations, were generally 2- to 4-fold lower in epithelium than in stroma. With finasteride concentrations greater than 5 nM, competitive inhibition of 5 alpha-reductase occurred both in epithelium and stroma. The mean inhibition constant Ki[nM +/- SEM] was 7 +/- 3 and 31 +/- 3 in epithelium and stroma, respectively. In the presence of finasteride concentrations < or = 5 nM, the epithelial 5 alpha-reductase seems to be inhibited in an uncompetitive manner, whereas such low finasteride concentrations cause either no inhibition (1-2 nM) or competitive inhibition (5 nM) in stroma. Our present study provides evidence that the inhibitory effect of finasteride on 5 alpha-reductase is much stronger in epithelium than in stroma. Therefore, it is conceivable that the global size-reduction of BPH under finasteride treatment is primarily due to the regression of BPH epithelium.
=============================================================
8.) Pharmacological treatment of benign prostatic hyperplasia with finasteride: a clinical
review.
=============================================================
Arch Esp Urol 1994 Nov;47(9):883-7; discussion 887-8
Ekman P
Department of Urology, Karolinska Hospital, Stockholm, Sweden.
Finasteride acts by blocking the conversion of testosterone to 5 alpha-dihydrotestosterone, the active androgen metabolite in the human prostate. In large, double-blind, placebo-controlled phase III studies recruiting over 1600 patients, it was shown that the administration of Finasteride, either 5 or 1 mg a day, reduced the size of the prostate by a mean of 22%, following 6 months of therapy. Despite this reduction in prostate size, urinary flow rate only improved by a mean of 1.7 ml per second and symptom score improved only marginally, but statistically significantly different from placebo. Long-term results in small series of patients have indicated a further improvement beyond 1 year. After 3 years flow was improved by 60%. The future role for Finasteride therapy is emerging, but it appears as if patients with mild to moderate symptoms would be a group who could benefit the most. Whether or not Finasteride can stop the long-term natural course of benign prostatic hyperplasia has still to be demonstrated.
=============================================================
9.) Medical therapy for benign prostatic hyperplasia: A review of the literature.
=============================================================
Eur Urol 2000 Jul;38(1):2-19
Clifford GM, Farmer RD
Public Health and Primary Care Research Unit, European Institute of Health and Medical
Sciences, University of Surrey, Surrey Research Park, UK.
[Medline record in process]
OBJECTIVE: To review the existing evidence regarding the efficacy and safety of medical therapy for lower urinary tract symptoms (LUTS) indicative of benign prostatic hyperplasia (BPH). To assess randomised controlled trials investigating the six alpha-adrenergic receptor antagonists (alpha-blockers), prazosin, alfuzosin, indoramin, terazosin, doxazosin, and tamsulosin, that benefit patients by relaxing prostatic smooth muscle, and the anti-androgen, finasteride, that mediates its more long-term benefits by reducing prostate size.
RESULTS:
This review suggests that both classes of drug offer significant improvement in criteria used to evaluate symptomatic BPH and can be effective whilst being acceptably safe. Furthermore, the therapeutic efficacy of all contemporary alpha-blockers appear similar, both in terms of symptom relief and urodynamic improvements. Randomised controlled trials have additionally demonstrated that finasteride therapy can provide improvement in terms of quality of life indices, prostate volume, and risks of progressing to acute urinary retention or prostatic surgery. While alpha-blockers have a rapid onset of action, likely to produce a therapeutic result within weeks, regardless of whether prostatic enlargement or bladder outlet obstruction is present, finasteride appears to be effective for more long-term therapy for up to 4 years, but only in alleviating symptoms when they are associated with a significantly large prostate. Neither finasteride nor the alpha(1a)-receptor-selective blocker, tamsulosin, are associated with the lowering of blood pressure and incidence of cardiovascular side effects that are apparent with other less selective alpha-blocker therapies such as dizziness and postural hypertension. They are, however, both associated with an increased risk of sexual dysfunction, albeit less than those associated with surgical intervention. Whereas tamsulosin is associated only with ejaculatory dysfunction, finasteride is additionally linked to decreased libido and impotence.
=============================================================
10.) Pretreatment with finasteride decreases perioperative bleeding associated with
transurethral resection of the prostate.
=============================================================
Urology 2000 May;55(5):684-9
Hagerty JA, Ginsberg PC, Harmon JD, Harkaway RC
Department of Surgery, Division of Urology, Albert Einstein Medical Center and
Philadelphia College of Osteopathic Medicine, Philadelphia, Pennsylvania, USA.
OBJECTIVES: The efficacy of finasteride in the treatment of gross hematuria associated with benign prostatic hyperplasia is well established. We evaluated a regimen of pretreatment with finasteride in decreasing perioperative bleeding associated with transurethral resection of the prostate (TURP). METHODS: A prospective analysis compared 25 patients pretreated with finasteride for 2 to 4 months before TURP with 50 patients without pretreatment. Patients in each group were further separated by the amount of prostate tissue resected. Patients were then followed up for perioperative bleeding, defined as a perioperative blood transfusion requirement or a return visit to the emergency room with gross hematuria or clot retention.
RESULTS: None of the patients with less than 30 g of prostate tissue resected experienced perioperative bleeding. In patients with 30 g or more resected, several episodes of bleeding occurred. In the patients pretreated with finasteride, 1 (8.3%) of 12 experienced perioperative bleeding; in the control group, 7 (36.8%) of 19 had an episode of bleeding. CONCLUSIONS: In patients with large prostate glands undergoing TURP, pretreatment with finasteride appears useful in reducing perioperative bleeding.
=============================================================
11.) Urinary retention in patients with BPH treated with finasteride or placebo over 4
years. Characterization of patients and ultimate outcomes.The PLESS Study Group.
=============================================================
Eur Urol 2000 May;37(5):528-36
Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox C, Anderson R, Kandzari
S, Herlihy R, Kornitzer G, Brown BT, Holtgrewe HL, Taylor A, Wang D, Waldstreicher
J
The University of Texas Southwestern Medical Center, Dallas, TX 07525-9110, USA.
claus.roehrborn@email.swmed.edu
OBJECTIVES: Knowledge regarding the incidence and prevalence of acute urinary retention and the ultimate outcome is very limited. The purpose of the present analysis was to document the natural history and outcomes of acute urinary retention (AUR) further specified as being either precipitated or spontaneous, and to evaluate the potential benefit of finasteride therapy.
MATERIALS AND METHODS:
Three thousand and forty men with moderate to severe symptoms of BPH and enlarged prostate glands by digital rectal examination were enrolled into the 4-year placebo-controlled PLESS trial and were evaluated for occurrences of AUR and BPH-related surgery. Men in the study were seen every 4 months; discontinued patients were followed up 6 months after discontinuation and again at the end of the 4-year trial. Complete 4-year data on outcomes (occurrence of AUR or BPH-related surgery) was available for 92% of the enrolled subjects in each treatment group. An endpoint committee, blinded to treatment group and center, reviewed and categorized all study-related documentation relating to retention and surgery.
RESULTS: Over the 4-year period, 99 of 1,503 placebo-treated patients (6.6%) experienced one or more episodes of AUR in comparison with 42 or 1,513 finasteride-treated patients (2.8%; p<0. 001). Approximately half of the episodes of retention were spontaneous and clearly BPH-related, while the other episodes were precipitated by another factor (PAUR). After spontaneous AUR, subsequent surgery was performed in 39 of 52 (75%) placebo-treated patients versus 8 of 20 (40%) finasteride-treated patients (p = 0. 01). BPH-related surgery was less common in men who had a prior episode of PAUR (26% in the placebo group and 14% in the finasteride group). CONCLUSION: There is a continual risk of spontaneous and precipitated acute urinary retention in men with moderate to severe lower urinary tract symptoms and an enlarged prostate gland. Fewer patients who developed precipitated AUR than spontaneous AUR go on to need subsequent BPH-related surgery. Significantly fewer finasteride-than placebo-treated patients developed AUR, and among those men, fewer ultimately needed BPH-related surgery.
=============================================================
12.) Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign
prostatic hyperplasia.
=============================================================
Eur Urol 2000 Apr;37(4):367-80
Bartsch G, Rittmaster RS, Klocker H
Department of Urology, University of Innsbruck, Austria. georg.bartsch@uibk.ac.at
OBJECTIVE:The development of the human benign prostatic hyperplasia clearly requires a combination of testicular androgens and aging. Although the role of androgens as the causative factor for human benign prostatic hyperplasia is debated, they undoubtedly have at least a permissive role. The principal prostatic androgen is dihydrotestosterone (DHT). Although not elevated in human benign prostatic hyperplasia, DHT levels in the prostate remain at a normal level with aging, despite a decrease in the plasma testosterone.
RESULTS:
DHT is generated by reduction of testosterone. Two isoenzymes of 5alpha-reductase have been discovered. Type 1 is present in most tissues of the body where 5alpha-reductase is expressed and is the dominant form in sebaceous glands. Type2 5alpha-reductase is the dominant isoenzyme in genital tissues, including the prostate. Finasteride is a 5alpha-reductase inhibitor that has been used for the treatment of benign prostatic hyperplasia and male-pattern baldness. At doses used clinically, its major effect is through suppression of type 2 5alpha-reductase, because it has a much lower affinity for the type 1 isoenzyme. Finasteride suppresses DHT by about 70% in serum and by as much as 85-90% in the prostate. The remaining DHT in the prostate is likely to be the result of type 1 5alpha-reductase.
Suppression of both 5alpha-reductase isoenzymes with GI198745 result in greater and more consistent suppression of serum dihydrotestosterone than that observed with a selective inhibitor of type 2 5alpha-reductase. Physiological and clinical studies comparing dual 5alpha-reductase inhibitors, such as GI198745, with selective type 2, such as finasteride, will be needed to determine the clinical relevance of type 1 5alpha-reductase within the prostate. Two large international multicenter, phase III trials have been published documenting the safety and efficacy of finasteride in the treatment of human benign prostatic hyperplasia. Combining these two studies, randomized, controlled data are available for 12 months. Noncontrolled extension of these data from a subset of patients, who elected to continue drug treatment for 3, 4 or 5 years, are also available. Long-term medical therapy with finasteride can reduce clinically significant endpoints such as acute urinary retention or surgery. According to the meta-analysis of six randomised clinical trial with finasteride, finasteride is most effective in men with large prostates. A more effective dual inhibitor of type 1 and 2 human 5alpha-reductase may lower circulating DHT to a greater extent than finasteride and show advantages in the treatment of human benign prostatic hyperplasia and other disease states that depend on DHT.
CONCLUSION:
Clinical evaluation of potent dual 5alpha-reductase inhibitors may help define the relative roles of human type 1 and 2 5alpha-reductase in the pathophysiology of benign prostatic hyperplasia and other androgen-dependent diseases.
=============================================================
13.) Chronic treatment with finasteride daily does not affect spermatogenesis or semen
production in young men.
=============================================================
J Urol 1999 Oct;162(4):1295-300 Related Articles, Books, LinkOut
Overstreet JW, Fuh VL, Gould J, Howards SS, Lieber MM, Hellstrom W, Shapiro S, Carroll P, Corfman RS, Petrou S, Lewis R, Toth P, Shown T, Roy J, Jarow JP, Bonilla J, Jacobsen CA, Wang DZ, Kaufman KD
Department of Obstetrics and Gynecology, University of California, Davis, USA.
PURPOSE:
Finasteride, an oral type 2, 5alpha-reductase inhibitor, is used in 1 mg. daily doses for the treatment of male pattern hair loss. A dose of 5 mg. finasteride daily reduces ejaculate volume by approximately 25%, and reduces prostate volume by approximately 20% and serum prostate specific antigen (PSA) by approximately 50% in men with benign prostatic hyperplasia. To our knowledge no data exist on the effect of 1 mg. finasteride daily on ejaculate volume or other semen parameters, or on the prostate in young men. Therefore, we studied the potential effect and reversibility of effect of 1 mg. finasteride daily on spermatogenesis, semen production, the prostate and serum PSA in young men.
MATERIALS AND METHODS:
In this double-blind, placebo controlled multicenter study 181 men 19 to 41 years old were randomized to receive 1 mg. finasteride or placebo for 48 weeks followed by a 60-week off-drug period. Of the 181 men 79 were included in a subset for the collection and analysis of sequential semen samples. RESULTS: There were no significant effects of 1 mg. finasteride on sperm concentration, total sperm per ejaculate, sperm motility or morphology. Ejaculate volume in subjects on finasteride decreased 0.3 ml. (-11%) compared to a decrease of 0.2 ml. (-8%) for placebo, with a median between treatment group difference of -0.03 ml. (1%, 90% confidence interval -10.4 to 13.1, p = 0.915). There were significant but small decreases in prostate volume (-2.6%) and serum PSA (-0.2 ng./ml.) in the finasteride group, which reversed on discontinuation of the drug.
CONCLUSIONS:
Treatment with 1 mg. finasteride daily for 48 weeks did not affect spermatogenesis or semen production in young men. The effects of 1 mg. finasteride daily on prostate volume and serum PSA in young men without benign prostatic hyperplasia were small and reversible on discontinuation of the drug.
=============================================================
14.) Management of androgenetic alopecia.
=============================================================
J Eur Acad Dermatol Venereol 1999 May;12(3):205-14 Related Articles, Books,
LinkOut
Tosti A, Camacho-Martinez F, Dawber R
Department of Dermatology, University of Bologna, Italy. tosti@almadns.unibo.it
BACKGROUND:
Androgenetic alopecia (AGA) is the most frequent cause of hair loss affecting up to 50% of men and 40% of women by the age of 50.
METHODS:
This paper outlines the current status of diagnosis and offers guidelines for optimal management of AGA in both men and women.
RESULTS:
The diagnosis of AGA can usually be confirmed by medical history and physical examination alone. A trichogram can be useful to assess the progression of the hair loss. A scalp biospy is diagnostic but usually not required. In women with signs of hyperandrogenism, investigation for ovarian (polycystic ovarian disease) or adrenal (late-onset congenital adrenal hyperplasia) disorders is required. Mild to moderate AGA in men can be treated with oral finasteride or topical minoxidil. Oral finasteride at the dosage of 1 mg/day produced clinical improvement in up to 66% of patients treated for 2 years. The drug is effective for both frontal and vertex hair thinning. Medical treatment with finasteride or minoxidil should be continued indefinitely since interruption of therapy leads to hair loss with return to pretreatment status. Mild to moderate AGA in women can be treated with oral antiandrogens (cyproterone acetate, spironolactone) and/or topical minoxidil with good results in many cases. Hair systems and surgery may be considered for selected cases of severe AGA both in men and in women.
CONCLUSIONS:
Patients with AGA should be informed about the pathogenesis of the condition. If used correctly, available medical treatments arrest progression of the disease and reverse miniaturization in most patients with mild to moderate AGA.
=============================================================
15.) Finasteride in the treatment of men with frontal male pattern hair loss.
=============================================================
J Am Acad Dermatol 1999 Jun;40(6 Pt 1):930-7 Related Articles, Books, LinkOut
Leyden J, Dunlap F, Miller B, Winters P, Lebwohl M, Hecker D, Kraus S, Baldwin H,
Shalita A, Draelos Z, Markou M, Thiboutot D, Rapaport M, Kang S, Kelly T, Pariser D,
Webster G, Hordinsky M, Rietschel R, Katz HI, Terranella L, Best S, Round E,
Waldstreicher J
University of Pennsylvania School of Medicine, Philadelphia, USA.
BACKGROUND:
Finasteride, a specific inhibitor of type II 5alpha-reductase, decreases serum and scalp dihydrotestosterone and has been shown to be effective in men with vertex male pattern hair loss.
OBJECTIVE:
This study evaluated the efficacy of finasteride 1 mg/day in men with frontal (anterior/mid) scalp hair thinning. METHODS: This was a 1-year, double-blind, placebo-controlled study followed by a 1-year open extension. Efficacy was assessed by hair counts (1 cm2 circular area), patient and investigator assessments, and global photographic review.
RESULTS:
There was a significant increase in hair count in the frontal scalp of finasteride-treated patients (P < .001), as well as significant improvements in patient, investigator, and global photographic assessments. Efficacy was maintained or improved throughout the second year of the study. Finasteride was generally well tolerated. CONCLUSION: In men with hair loss in the anterior/mid area of the scalp, finasteride 1 mg/day slowed hair loss and increased hair growth.
=============================================================
16.) Androgenetic alopecia in men: the scale of the problem and prospects for treatment.
=============================================================
Int J Clin Pract 1999 Jan-Feb;53(1):50-3 Related Articles, Books, LinkOut
Rushton DH
School of Pharmacy and Biomedical Sciences, University of Portsmouth, Hants.
While the precise incidence of androgenetic alopecia is unknown, it is universally acknowledged to be the most common hair problem in men. Balding is generally associated with ageing; consequently, the desire to prolong a youthful appearance inevitably leads to demands for effective treatments. Further, changing attitudes in modern society have resulted in people becoming concerned about their appearance and less tolerant about conditions that might be alleviated by medical intervention. The importance of hair loss upon quality of life has been underestimated by the medical profession. Clinicians failing to accept hair loss as an important medical problem ignore the real distress suffered by a significant proportion of those affected. New options for treatment that selectively target the metabolic pathways involved in the balding process are showing promise. The first generation of such drugs, Propecia, is now available in some countries and other molecules are currently under development.
=============================================================
17.) [Androgenetic alopecia, hirsutism and hypertrichosis].
=============================================================
Ther Umsch 1999 Apr;56(4):219-24 Related Articles, Books, LinkOut
Trueb RM, Wyss M, Itin PH
Dermatologische Klinik, Universitatsspital Zurich.
Having too much hair on the face or the body and not enough on the scalp respectively, is generally not a mirror of a life-threatening disease. However, the emotional impact of such cosmetic problems may be remarkable in the individual case. Currently rational treatment options are becoming increasingly available to correct such hair problems. This review highlights the new therapeutic achievements in the treatment of both androgenetic alopecia and hirsutism. Oral treatment of male patterned hair loss with finasteride in men is emphasized, and the use of antiandrogens in women is discussed. In addition, the mode of action and clinical results of topical minoxidil treatment find mention. The second part of the review deals with hirsutism and hypertrichosis. The diagnostic steps and investigations are briefly reviewed, and the advances in laser treatment of hirsutism and hypertrichosis are presented.
============================================================= BR>118.) Medical treatments for balding in men.
=============================================================
Am Fam Physician 1999 Apr 15;59(8):2189-94, 2196 Related Articles, Books,
LinkOut
Scow DT, Nolte RS, Shaughnessy AF
Harrisburg Family Practice Residency, PA 17105-8700, USA.
Two drugs are available for the treatment of balding in men. Minoxidil, a topical product, is available without a prescription in two strengths. Finasteride is a prescription drug taken orally once daily. Both agents are modestly effective in maintaining (and sometimes regrowing) hair that is lost as a result of androgenic alopecia. The vertex of the scalp is the area that is most likely to respond to treatment, with little or no hair regrowth occurring on the anterior scalp or at the hairline. Side effects of these medications are minimal, making them suitable treatments for this benign but psychologically disruptive condition.
============================================================= 19.) Understanding and managing common baldness.
=============================================================
Aust Fam Physician 1999 Mar;28(3):248-50, 252-3
Tran D, Sinclair RD
Department of Dermatology, St Vincent's Hospital, Melbourne.
BACKGROUND:
Society places importance on physical attributes especially the appearance of our hair. Common baldness or androgenetic alopecia is a normal physiological process of hair loss in genetically predisposed individuals. Premature or accelerated hair loss can engender considerable negative thoughts and anxiety associated with feelings of diminished attractiveness. OBJECTIVE: To enable general practitioners to recognise the various treatment options available, therefore offering patients reasonable hope and informed choices.
DISCUSSION:
Common baldness can be prevented by currently available mediums and regrowth may be achieved in a significant percentage of cases. Correct use of these agents requires an understanding of the pathogenesis of androgenetic alopecia, its natural history and the time course of response to treatment.
=============================================================
20.) Finasteride: a review of its use in male pattern hair loss.
=============================================================
Drugs 1999 Jan;57(1):111-26 Related Articles, Books, LinkOut
McClellan KJ, Markham A
Adis International Limited, Mairangi Bay, Auckland, New Zealand. demail@adis.co.nz
The 5alpha-reductase inhibitor finasteride blocks the conversion of testosterone to dihydrotestosterone (DHT), the androgen responsible for male pattern hair loss (androgenetic alopecia) in genetically predisposed men. Results of phase III clinical studies in 1879 men have shown that oral finasteride 1 mg/day promotes hair growth and prevents further hair loss in a significant proportion of men with male pattern hair loss.
Evidence suggests that the improvement in hair count reported after 1 year is maintained during 2 years' treatment. In men with vertex hair loss, global photographs showed improvement in hair growth in 48% of finasteride recipients at 1 year and in 66% at 2 years compared with 7% of placebo recipients at each time point. Furthermore, hair counts in these men showed that 83% of finasteride versus 28% of placebo recipients had no further hair loss compared with baseline after 2 years. The clinical efficacy of oral finasteride has not yet been compared with that of topical minoxidil, the only other drug used clinically in patients with male pattern hair loss. Therapeutic dosages of finasteride are generally well tolerated. In phase III studies, 7.7% of patients receiving finasteride 1 mg/day compared with 7.0% of those receiving placebo reported treatment-related adverse events.
The overall incidence of sexual function disorders, comprising decreased libido, ejaculation disorder and erectile dysfunction, was significantly greater in finasteride than placebo recipients (3.8 vs 2.1%). All sexual adverse events were reversed on discontinuation of therapy and many resolved in patients who continued therapy. No other drug-related events were reported with an incidence > or =1% in patients receiving finasteride. Most events were of mild to moderate severity. Oral finasteride is contraindicated in pregnant women because of the risk of hypospadias in male fetuses.
CONCLUSIONS:
Oral finasteride promotes scalp hair growth and prevents further hair loss in a significant proportion of men with male pattern hair loss. With its generally good tolerability profile, finasteride is a new approach to the management of this condition, for which treatment options are few. Its role relative to topical minoxidil has yet to be determined.
=============================================================
21.) Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male
Pattern Hair Loss Study Group.
=============================================================
J Am Acad Dermatol 1998 Oct;39(4 Pt 1):578-89
Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, Price VH, Van
Neste D, Roberts JL, Hordinsky M, Shapiro J, Binkowitz B, Gormley GJ
Department of Clinical Research, Merck Research Laboratories, Rahway, NJ 07065,
USA. /font>
BACKGROUND:
Androgenetic alopecia (male pattern hair loss) is caused by androgen-dependent miniaturization of scalp hair follicles, with scalp dihydrotestosterone (DHT) implicated as a contributing cause. Finasteride, an inhibitor of type II 5alpha-reductase, decreases serum and scalp DHT by inhibiting conversion of testosterone to DHT.
OBJECTIVE:
Our purpose was to determine whether finasteride treatment leads to clinical improvement in men with male pattern hair loss.
METHODS:
In two 1-year trials, 1553 men (18 to 41 years of age) with male pattern hair loss received oral finasteride 1 mg/d or placebo, and 1215 men continued in blinded extension studies for a second year. Efficacy was evaluated by scalp hair counts, patient and investigator assessments, and review of photographs by an expert panel. RESULTS: Finasteride treatment improved scalp hair by all evaluation techniques at 1 and 2 years (P < .001 vs placebo, all comparisons). Clinically significant increases in hair count (baseline = 876 hairs), measured in a 1-inch diameter circular area (5.1 cm2) of balding vertex scalp, were observed with finasteride treatment (107 and 138 hairs vs placebo at 1 and 2 years, respectively; P < .001). Treatment with placebo resulted in progressive hair loss. Patients' self-assessment demonstrated that finasteride treatment slowed hair loss, increased hair growth, and improved appearance of hair. These improvements were corroborated by investigator assessments and assessments of photographs. Adverse effects were minimal.
CONCLUSION:
In men with male pattern hair loss, finasteride 1 mg/d slowed the progression of hair loss and increased hair growth in clinical trials over 2 years.
=============================================================
22.) Genetic analysis of male pattern baldness and the 5alpha-reductase genes.
=============================================================
J Invest Dermatol 1998 Jun;110(6):849-53 Related Articles, Books, LinkOut
Ellis JA, Stebbing M, Harrap SB
Department of Physiology, The University of Melbourne, Parkville, Victoria, Australia.
Genetic predisposition and androgen dependence are important characteristics of the common patterned loss of scalp hair known as male pattern baldness. The involvement of the 5alpha-reductase enzyme in male pattern baldness has been postulated due to its role in the metabolism of testosterone to dihydrotestosterone.
There are two known isozymes of 5alpha-reductase. Type I has been predominantly localized to the skin and scalp. Type II, also present on the scalp, is the target of finasteride, a promising treatment for male pattern baldness. We conducted genetic association studies of the 5alpha-reductase enzyme genes (SRD5A1 on chromosome 5 and SRD5A2 on chromosome 2) using dimorphic intragenic restriction fragment length polymorphisms. >From a population survey of 828 healthy families comprising 3000 individuals, we identified 58 young bald men (aged 18-30 y) and 114 older nonbald men (aged 50-70 y) for a case control comparison.
No significant differences were found between cases and controls in allele, genotype, or haplotype frequencies for restriction fragment length polymorphisms of either gene. These findings suggest that the genes encoding the two 5alpha-reductase isoenzymes are not associated with male pattern baldness. Finally, no clear inheritance pattern of male pattern baldness was observed. The relatively strong concordance for baldness between fathers and sons in this study was not consistent with a simple Mendelian autosomal dominant inheritance. A polygenic etiology should be considered.
=============================================================
23.) Effect of finasteride on human testicular steroidogenesis.
=============================================================
J Androl 1996 Sep-Oct;17(5):516-21 Related Articles, Books
Castro-Magana M, Angulo M, Fuentes B, Canas A, Sarrantonio M, Arguello R, Vitollo
P
Department of Pediatrics, Winthrop-University Hospital, Mineola, New York 11501,
USA.
We studied the testicular function and some androgen-mediated events in 22 males (16-30 years of age) with male pattern baldness that was treated with finasteride (10 mg once daily) for 2 years. Patients were evaluated every 3 months. Prostatic volume was determined in six subjects by endorectal ultrasound scans.
Serum gonadotropin, prostate-specific antigen (PSA), and sex hormone levels were determined basally and periodically during the treatment period. Fourteen subjects underwent gonadal stimulation with human chorionic gonadotropin (hCG), and the gonadotropin response to gonadotropin releasing hormone (GnRH) was determined in eight subjects, prior to and after 2 years of therapy.
Finasteride treatment resulted in an improvement in the male pattern baldness and prostatic shrinkage that was associated with an increase in serum testosterone levels (17.2 +/- 2.5 vs. 26.3 +/- 1.7 nmol/L) and a decrease in dihydrotestosterone (DHT) levels (1.45 +/- 0.41 vs. 0.38 +/- 0.10 nmol/L), causing a marked increase in that testosterone/DHT ratio. A significant increase in the serum levels of androstenedione (3.67 +/- 0.49 vs. 7.05 +/- 0.70 nmol/L) and estradiol (132 +/- 44 vs. 187 +/- 26 pmol/L) was also noted, whereas androstanediol glucoronide (33.3 +/- 6.4 vs. 10.7 +/- 4.5 pmol) and PSA (1.6 +/- 0.6 vs. 0.4 +/- 0.1 ng/ml) were significantly decreased. No changes in basal or stimulated levels of gonadotropin were observed.
There was a significant increase in the testosterone response to hCG during finasteride therapy (delta: 16.7 vs. 35.5 nmol/L) that could be explained, at least in part, by the reduction of testosterone metabolism resulting from the blockage induced by finasteride. The decrease in the androstenedione to testosterone and estrone to estradiol ratios observed after hCG treatment, however, strongly suggests increased activity of the 17-ketosteroid reductase enzyme and an improvement of the testicular capacity for testosterone production.
============================================================= BR>224.) [Finasteride: a new drug for the treatment of male hirsutism and androgenetic
alopecia]?
=============================================================
Clin Ter 1996 Jun;147(6):305-15 Related Articles, Books, LinkOut
[Article in Italian]
Spinucci G, Pasquali R
Dipartimento di Medicina interna e Gastroenterologia, Policlinico S. Orsola-Malpighi,
Bologna.
Finasteride is a drug which inhibits the transformation of testosterone into its active metabolite, dihydrotestosterone, in the target organs, i.e. the skin, the scalp, the liver and the prostate. In the pathogenic mechanism of hirsutism and androgenetic alopecia, and important role is presumably played by alterations of the mechanisms which transform testosterone into dihydrotestosterone. In some conditions an increase in dihydrotestosterone has been demonstrated, due to increased activity of the enzyme 5 alpha-reductase.
The effect of finasteride develops above all at the level of type II 5 alpha-reductase. Recent studies have evaluated the effect of finasteride in patients of both sexes with hirsutism and androgenetic alopecia. In women with various forms of hyperandrogenism, the use of the drug at the doses commonly used for the treatment of benign prostatic hyperplasia seems to have induced a significant reduction in the degree of hirsutism.
Furthermore, both in animals and men with alopecia, the drug seems to have led to an increase in the number and an improvement in the shape of the follicles in the anagen phase, and a simultaneous decrease of dehydrotestosterone at the level of the scalp. This study represents a review of the main results obtained over the last two years and reports the prospects which the use of finasteride may have in this context.
=============================================================
25.) The 5 alpha-reductase system and its inhibitors. Recent development and its
perspective in treating androgen-dependent skin disorders.
=============================================================
Dermatology 1996;193(3):177-84 Related Articles, Books, LinkOut
Chen W, Zouboulis CC, Orfanos CE
Department of Dermatology, University Medical Center Benjamin Franklin, Free
University of Berlin, Germany.
5 alpha-Reductase, the enzyme system that metabolizes testosterone into dihydrotestosterone, occurs in two isoforms. The type 1 isozyme is composed of 259 amino acids, has an optimal pH of 6-9 and represents the 'cutaneous type'; it is located mainly in sebocytes but also in epidermal and follicular keratinocytes, dermal papilla cells and sweat glands as well as in fibroblasts from genital and non-genital skin. The type 2 isozyme is composed of 254 amino acids, has an optimal pH of about 5.5 and is located mainly in the epididymis, seminal vesicles, prostate and fetal genital skin as well as in the inner root sheath of the hair follicle and in fibroblasts from normal adult genital skin. The genes encoding type 1 and type 2 isozymes are found in chromosomes 5p and 2p, respectively, and each consists of 5 exons and 4 introns.
During the last decade, several steroid analogues and non-steroid agents have been developed to interfere with 5 alpha-reductase activity. Finasteride, which has a higher affinity for the type 2 isozyme, is the first 5 alpha-reductase antagonist clinically introduced for treatment of benign prostate hyperplasia. The clinical evaluation of finasteride or other 5 alpha-reductase inhibitors in the field of dermatology has been very limited; in particular, those that selectively bind to type 1 isozyme (e.g. MK-386, LY191704) may be regarded as candidates for treatment of androgen-dependent skin disorders such as seborrhoea, acne, hirsutism and/or androgenetic alopecia.
=============================================================
26.) Finasteride: a clinical review.
=============================================================
Biomed Pharmacother 1995;49(7-8):319-24 Related Articles, Books
Gormley GJ
Merck Research Laboratories, Rahway, NJ 07065-0914, USA.
Finasteride is the first of a new class of 5 alpha-reductase inhibitors which allows selective androgen deprivation affecting dihydrotestosterone (DHT) levels in target organs such as the prostate and scalp hair without effecting circulating levels of testosterone thus preserving the desired androgen mediated effects on muscle strength, bone density and sexual function. Finasteride has been demonstrated to produce significant effects in men with an enlarged prostate gland. The long-term data now emerging suggests that progression of benign prostatic hyperplasia (BPH) may be arrested providing additional long term benefits. Experimental uses in prostate cancer prevention and male pattern baldness offer new and exciting possibilities for this class of compounds.
=============================================================
27.) The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and
dihydrotestosterone concentrations in patients with male pattern baldness.
=============================================================
J Clin Endocrinol Metab 1994 Sep;79(3):703-6 Related Articles, Books, LinkOut
Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, Nguyen HH,
Moore EC, Tanaka WK
Merck Research Laboratories, Rahway, New Jersey 07065.
The effects of the 5 alpha-reductase inhibitor, finasteride, on scalp skin testosterone (T) and dihydrotestosterone (DHT) levels were studied in patients with male pattern baldness. In a double blind study, male patients undergoing hair transplantation were treated with oral finasteride (5 mg/day) or placebo for 28 days. Scalp skin biopsies were obtained before and after treatment for measurement of T and DHT by high pressure liquid chromatography-RIA. In 10 male subjects studied at baseline, mean (+/- SEM) DHT levels were significantly higher in bald (7.37 +/- 1.24 pmol/g) compared to hair-containing (4.20 +/- 0.65 pmol/g) scalp, whereas there was no difference in mean T levels at baseline. In bald scalp from 8 patients treated with finasteride, the mean DHT concentration decreased from 6.40 +/- 1.07 pmol/g at baseline to 3.62 +/- 0.38 pmol/g on day 28. Scalp T levels increased in 6 of 8 subjects treated with finasteride.
Finasteride decreased the mean serum DHT concentration from 1.36 +/- 0.18 nmol/L (n = 8) at baseline to 0.46 +/- 0.10 nmol/L on day 28 and had no effect on serum T. There were no significant changes in scalp or serum T or DHT in placebo-treated patients. In this study, male subjects treated with 5 mg/day finasteride for 4 weeks had significantly decreased concentrations of DHT in bald scalp, resulting in a mean level similar to the baseline levels found in hair-containing scalp.
=============================================================
28.) Finasteride: the first 5 alpha-reductase inhibitor.
=============================================================
Pharmacotherapy 1993 Jul-Aug;13(4):309-25; discussion 325-9 Related Articles,
Books
Sudduth SL, Koronkowski MJ
Program on Aging, School of Pharmacy, University of North Carolina, Chapel Hill
27599-7360.
Finasteride is a synthetic 4-azasteroid that is a specific competitive inhibitor of 5 alpha-reductase, an intracellular enzyme that converts testosterone to dihydrotestosterone (DHT). It has no binding affinity for androgen receptor sites and itself possesses no androgenic, antiandrogenic, or other steroid hormone-related properties. It is well absorbed after oral administration, with absolute bioavailability in humans of 63% (range 34-108%). The mean time to maximum concentration is 1-2 hours, and it is approximately 90% plasma protein bound. The elimination half-life averages 6-8 hours.
The agent is metabolized to a series of five metabolites, of which two are active and possess less than 20% of the 5 alpha-reductase activity of finasteride. Little is known about potential drug interactions, although they appear to be minimal and not clinically relevant. The drug is indicated for the treatment of symptomatic benign prostatic hyperplasia. Its efficacy in regression of prostate gland enlargement is rapid and predictable, although correlation with subsequent improvement in urinary flow and symptoms is highly variable.
Dosages of 0.5-100 mg/day regress prostate enlargement; the recommended dosage is 5 mg once/day. Finasteride may hold promise for other DHT-mediated disorders such as acne, facial hirsutism, frontal lobe alopecia, and prostate cancer, but its use in these conditions remains investigational.
The frequency of adverse drug events is low, with the most common side effects being impotence, decreased libido, and decreased volume of ejaculate. No reports of intentional overdose have been reported, and dosages of up to 80 mg/day for 3 months have been taken without adverse effect.
=============================================================
29.) Cytologic atypia in a 53-year-old man with finasteride-induced gynecomastia.
=============================================================
Arch Pathol Lab Med 2000 Apr;124(4):625-7 Related Articles, Books, LinkOut
Zimmerman RL, Fogt F, Cronin D, Lynch R
Departments of Pathology & Laboratory Medicine, Presbyterian Medical Center,
University of Pennsylvania Health System, Philadelphia, PA 19104, USA.
Finasteride has been associated with the development of gynecomastia. Although cytoplasmic vacuolization has been noted in prostatic epithelium in men taking this drug, we found no documentation of the cytologic changes in finasteride-associated gynecomastia. We present the case of a 53-year-old man who developed unilateral gynecomastia following finasteride therapy for alopecia. A fine-needle aspiration biopsy of the mass was diagnosed as adenocarcinoma on the basis of nuclear atypia and particularly because of cytoplasmic vacuolization.
Subsequent excisional biopsy revealed benign gynecomastia with no evidence of malignant change. The ductal epithelium did exhibit cytoplasmic vacuolization similar to that described in the prostate following finasteride therapy. We believe this is the first reported case documenting the cytologic changes seen in gynecomastia secondary to finasteride therapy. Cytoplasmic vacuolization in this setting should not be considered evidence of malignancy in men with gynecomastia. As with gynecomastia in general, extreme caution should be used before rendering a cytologic diagnosis of malignancy.
=============================================================
30.) Reversible painful gynaecomastia induced by low dose finasteride (1 mg/day).
=============================================================
Australas J Dermatol 2000 Feb;41(1):55 Related Articles, Books, LinkOut
Wade MS, Sinclair RD
Publication Types:
Letter
============================================================= /font>
==============================================================
31.) Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts
in horizontal sections of serial scalp biopsies: results of finasteride 1 mg treatment of men
and postmenopausal women.
=============================================================
J Investig Dermatol Symp Proc 1999 Dec;4(3):282-4 Related Articles, Books, LinkOut
Whiting DA, Waldstreicher J, Sanchez M, Kaufman KD
Baylor Hair Research and Treatment Center, Baylor University Medical Center, Dallas,
Texas 75246, USA.
Hair regrowth was evaluated by histologic analysis in men and women treated for androgenetic alopecia, by counting follicles in horizontal sections of scalp biopsies. Serial 4mm punch biopsies were taken at baseline and after 12mo of treatment from the transitional area of hair thinning between normal hair and vertex balding in men, and in an area of frontal/parietal thinning in women. Horizontal sections of reticular and papillary dermis were read by one observer, blinded to patient, treatment, and time. All terminal hair bulbs, terminal anagen and telogen hairs, and vellus and vellus-like miniaturized hairs were counted.
Twenty-six men aged 18-41y, comprising 14 on finasteride 1 mg daily and 12 on placebo, and 94 postmenopausal women, aged 41-60y, comprising 44 on finasteride 1 mg daily and 50 on placebo, were evaluated. In the male study, the terminal hairs increased from a mean baseline count of 15.5-20.9 after 12mo of finasteride, versus 17.3-18.3 in the placebo patients.
The miniaturized hairs decreased from 26.7 to 23.6 with finasteride versus 21.3-20.3 with placebo. The terminal-to-vellus ratio increased more in the finasteride than in the placebo patients, suggesting some reversal of the miniaturization process with finasteride. In the female study, no significant differences in follicular counts were found between the finasteride and placebo groups after 12mo of treatment. Follicular counts in horizontal sections provide an informative adjunct to noninvasive measures used in hair growth studies. Finasteride appears to be capable of reversing hair miniaturization in androgenetic alopecia in young to middle-aged men, but not in postmenopausal women.
=============================================================
32.) Improvement in androgenetic alopecia in 53-76-year-old men using oral finasteride.
=============================================================
Int J Dermatol 1999 Dec;38(12):928-30 Related Articles, Books, LinkOut
Brenner S, Matz H
Department of Dermatology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Twenty-eight men with AGA, aged 53-76 years (mean, 65 years), were selected to participate in this trial from a double blind, placebo controlled, multicenter study of subjects with moderate symptoms of BPH. Patients received either finasteride 5 mg or placebo daily for 24 months. Hair counts were performed at entry to the study and at 6, 12, 18, and 24 months. Hair counts were made directly on the scalp in a circular target area 1 in in diameter, located in the center of a template. The template was applied in such a way that its counting window fell on the most balding scalp area, which remained the same for each patient.11 At each hair counting session, patients were asked about side-effects and questioned about their sex life. Time trend and differences between groups were examined using a one-way (treatment) MANOVA with repeated measures (baseline, 6, 12, 18, and 24 months). Additional two-tailed t-tests were performed to compare the two groups at each point of time. P < 0.05 was considered to be significant.
=============================================================
33.) New topical antiandrogenic formulations can stimulate hair growth in human bald
scalp grafted onto mice.
=============================================================
Int J Pharm 2000 Jan 20;194(1):125-34 Related Articles, Books, LinkOut
Sintov A, Serafimovich S, Gilhar A
Ben-Gurion University of the Negev, The Institutes for Applied Research, PO Box 653,
Beer-Sheva, Israel. asintov@bgumail.bgu.ac.il
The purpose of this study was to test the ability of topical formulations of finasteride and flutamide to re-enlarge hair follicles in male-pattern baldness. This was evaluated by an experimental model of human scalp skin graft transplanted onto SCID mice.
A comparison was made between formulations containing finasteride and flutamide, and a vehicle formulation in terms of the mean hairs per graft, length, diameter of the shafts, and structures of the growth stages of the hair. Flutamide and finasteride had a significantly higher effect (P<0.05) than the placebo in all the tested parameters, but flutamide demonstrated more hair per graft and longer hair shafts than finasteride (P<0.05). The number of hairs per graft for flutamide and finasteride groups were 1.22+/-0. 47 and 0.88+/-0.95 hairs/0.5 mm2 graft, respectively, versus 0. 35+/-0.6 hairs/graft for vehicle-treated graft. Similarly, hair lengths for flutamide and finasteride were 5.82+/-0.50 and 4.50+/-0. 32 mm, respectively, versus 2.83+/-0.18 mm for the vehicle-treated grafts.
An in vitro diffusion study of flutamide gel using hairless mouse skin demonstrated the beneficial effect of the vehicle composition in comparison with a hydroalcoholic solution or a gel containing no penetration enhancer. It is therefore suggested that this topical composition containing flutamide or finasteride may effectively result in regression of male-pattern baldness.
=============================================================
34.) Current management of androgenetic alopecia in men.
=============================================================
Eur J Dermatol 1999 Dec;9(8):606-9 Related Articles, Books, LinkOut
Wolff H, Kunte C
Klinik fur Dermatologie und Allergologie, Ludwig-Maximilians-Universitat, Frauenlobstrasse 9-11, D-80337, Munchen, Germany. hans.wolff@lrz.uni-muenchen.de
Androgenetic alopecia (AGA) is a common dermatological condition affecting both men and women. Until recently there has been little interest in AGA as a clinical condition, largely due to the lack of any genuinely effective treatment for it. A number of "remedies" exist, such as vitamin supplements, which are not generally harmful but which have no proven efficacy in promoting hair growth or preventing further hair loss. Hair systems and surgery provide camouflage for the symptoms but do not effect a cure. By far the most promising approaches to the treatment of AGA are drug therapies, such as minoxidil and finasteride. Finasteride, an inhibitor of the type II 5alpha-reductase that converts testosterone to dihydrotestosterone, has been shown to prevent further hair loss, and promotes new hair growth in the majority of the men taking part in clinical trials. Tailored drug approaches like this offer the greatest hope for the successful future treatment of alopecia.
=============================================================
35.) Immunohistochemical localization of types 1 and 2 5alpha-reductase in human scalp.
=============================================================
Br J Dermatol 1999 Sep;141(3):481-91 Related Articles, Books, LinkOut
Bayne EK, Flanagan J, Einstein M, Ayala J, Chang B, Azzolina B, Whiting DA, Mumford
RA, Thiboutot D, Singer II, Harris G
Merck Research Laboratories, PO Box 2000, Rahway, NJ 07065, USA.
ellen_bayne@merck.com
The predominant form of 5alpha-reductase (5aR) in human scalp is 5aR1. None the less, clinical studies have shown that finasteride, a selective inhibitor of 5aR2, decreases scalp dihydrotestosterone and promotes hair growth in men with androgenetic alopecia.
Immunolocalization studies were thus carried out to examine 5aR isozyme distribution within scalp and, in particular, to determine whether 5aR2 might be associated with hair follicles. 5aR2 was localized using both a rabbit polyclonal and a mouse monoclonal antibody. 5aR1 was detected with a mouse monoclonal antibody.
The specificity of these reagents was demonstrated both by immunofluorescence and Western blot analyses of COS cells overexpressing human 5aR1 or 5aR2. When cryosections of scalp from men with androgenetic alopecia were stained with antibody against 5aR2, using immunoperoxidase avidin-biotin complex methodology, immunostaining was observed in the inner layer of the outer root sheath and, in more proximal regions of the follicle, in the inner root sheath. Staining was also prominent in the infundibular region of the follicle, with less intense staining extending throughout the granular layer of the epidermis.
Some staining was also seen in sebaceous ducts. Similar results were obtained with both the polyclonal and monoclonal 5aR2 antibodies. In contrast, in scalp cryosections stained with antibody to 5aR1, no immunostaining was observed within hair follicles. Intense staining for the type 1 isozyme was, however, detected within sebaceous glands. Our immunolocalization data suggest that the results seen in clinical trials of men with male pattern hair loss treated with finasteride may be due, at least in part, to local inhibition of 5aR2 within the hair follicle.
=============================================================
36.) The psychosocial consequences of androgenetic alopecia: a review of the research
literature.
=============================================================
Br J Dermatol 1999 Sep;141(3):398-405 Related Articles, Books, LinkOut
Cash TF
Department of Psychology, Old Dominion University, Norfolk, VA 23529-0267, USA.
Androgenetic alopecia is a common dermatological condition, with potentially adverse psychosocial sequelae. The present review critically examines scientific evidence concerning the effects of androgenetic hair loss on social processes and psychological functioning, as well as the psychosocial outcomes of medical treatments. Research confirms a negative but modest effect of visible hair loss on social perceptions.
More importantly, androgenetic alopecia is typically experienced as a moderately stressful condition that diminishes body image satisfaction. Deleterious effects on self-esteem and certain facets of psychological adjustment are more apparent among women than men and among treatment-seeking patients. Various 'risk factors' vis-a-vis the psychological adversity of androgenetic alopecia are identified. Medical treatments, i.e. minoxidil and finasteride, appear to have some psychological efficacy.
A conceptual model is delineated to explain the psychological effects of hair loss and its treatment. Directions for needed research are discussed. Strategies are presented for the clinical management of psychological issues among these patients.
=============================================================
37.) Clinical dose ranging studies with finasteride, a type 2 5alpha-reductase inhibitor, in
men with male pattern hair loss.
=============================================================
J Am Acad Dermatol 1999 Oct;41(4):555-63 Related Articles, Books, LinkOut
Roberts JL, Fiedler V, Imperato-McGinley J, Whiting D, Olsen E, Shupack J, Stough D, DeVillez R, Rietschel R, Savin R, Bergfeld W, Swinehart J, Funicella T, Hordinsky M, Lowe N, Katz I, Lucky A, Drake L, Price VH, Weiss D, Whitmore E, Millikan L, Muller S, Gencheff C, et al
Northwest Cutaneous Research Specialists, Portland, Oregan, USA.
BACKGROUND:
Androgenetic alopecia is a common condition of adult men. Finasteride, a type 2 5alpha-reductase inhibitor, decreases the formation of dihydrotestosterone from testosterone. OBJECTIVE: Two separate clinical studies were conducted to establish the optimal dose of finasteride in men with this condition.
METHODS:
Men from 18 to 36 years of age with moderate vertex male pattern hair loss received finasteride 5, 1, 0.2, or 0.01 mg/day or placebo based on random assignment. Efficacy was determined by scalp hair counts, patient self-assessment, investigator assessment, and assessment of clinical photographs. Safety was assessed by clinical and laboratory measurements and by analysis of adverse experiences.
RESULTS:
Efficacy was demonstrated for all end points for finasteride at doses of 0.2 mg/day or higher, with 1 and 5 mg demonstrating similar efficacy that was superior to lower doses. Efficacy of the 0.01 mg dose was similar to placebo. No significant safety issues were identified in the trials.
CONCLUSION:
Finasteride 1 mg/day is the optimal dose for the treatment of men with male pattern hair loss and was subsequently identified for further clinical development.
============================================================= BR>38.) The effects of finasteride on scalp skin and serum androgen levels in men with
androgenetic alopecia.
=============================================================
J Am Acad Dermatol 1999 Oct;41(4):550-4 Related Articles, Books, LinkOut
Drake L, Hordinsky M, Fiedler V, Swinehart J, Unger WP, Cotterill PC, Thiboutot DM,
Lowe N, Jacobson C, Whiting D, Stieglitz S, Kraus SJ, Griffin EI, Weiss D, Carrington
P, Gencheff C, Cole GW, Pariser DM, Epstein ES, Tanaka W, Dallob A, Vandormael
K, Geissler L, Waldstreicher J
University of Oklahoma Health Sciences, Oklahoma City, USA.
BACKGROUND:
Data suggest that androgenetic alopecia is a process dependent on dihydrotestosterone (DHT) and type 2 5alpha-reductase. Finasteride is a type 2 5alpha-reductase inhibitor that has been shown to slow further hair loss and improve hair growth in men with androgenetic alopecia.
OBJECTIVE:
We attempted to determine the effect of finasteride on scalp skin and serum androgens.
METHODS:
Men with androgenetic alopecia (N = 249) underwent scalp biopsies before and after receiving 0.01, 0.05, 0.2, 1, or 5 mg daily of finasteride or placebo for 42 days. RESULTS: Scalp skin DHT levels declined significantly by 13.0% with placebo and by 14.9%, 61.6%, 56. 5%, 64.1%, and 69.4% with 0.01, 0.05, 0.2, 1, and 5 mg doses of finasteride, respectively. Serum DHT levels declined significantly (P <.001) by 49.5%, 68.6%, 71.4%, and 72.2% in the 0.05, 0.2, 1, and 5 mg finasteride treatment groups, respectively.
CONCLUSION:
In this study, doses of finasteride as low as 0.2 mg per day maximally decreased both scalp skin and serum DHT levels. These data support the rationale used to conduct clinical trials in men with male pattern hair loss at doses of finasteride between 0.2 and 5 mg.
===================================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No 2-(95) 25/08/2000 DR. JOSÉ LAPENTA R.
UPDATED 18 JULY 2025
===================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela 1.998-2.025
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.025
Tlf: 0414-2976087 - 04127766810















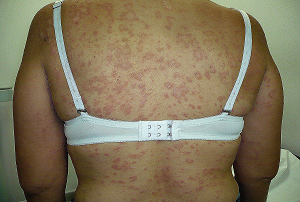




.png)