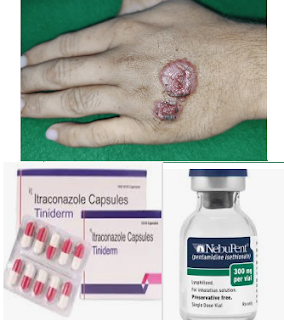
LEISHMANIASIS PENTAMIDINA ITRACONAZOL
Se hace una revisión Bibliográfica de tratamientos para LEISHMANIASIS CUTÁNEA con PENTAMIDINA e ITRACONAZOLE.
***********************************
EDITORIAL ESPANOL:
====================
Hola amigos, DERMAGIC los saluda, el tema de la revision de hoy: LEISHMANIASIS CUTANEA y su tratamiento con PENTAMIDINA E ITRACONAZOLE,
La semana pasada en la LISTA DERMLIST (BRASIL) se habló de este tema y de alli surgió la revisión.
Espero sean bastante interesantes para actualizarnos estas 26 REFERENCIAS biliográficas. Ultimamente se esta utilizando el INTERFERON GAMMA para su tratamiento (21,22). En BRAZIL y VENEZUELA se esta tratando de hacer una vacuna para esta patología (25,26)
Hasta una próxima edición,,, saludos
Próximas ediciones: * ESPOROTRICOSIS (I)
* ESPOROTRICOSIS (II)
* LA TOXINA BOTULÍNICA
EDITORIAL ENGLISH:
===================
Hello friends, DERMAGIC greets all, the topic of today's revision: CUTANEOUS LEISHMANIASIS and their treatment with PENTAMIDINA AND ITRACONAZOLE,
Last week in the LIST DERMLIST (BRAZIL) it was spoken of this topic, and of there the revision arose.
I wait they are quite interesting to modernize us, these 26 bibliographical REFERENCES. New alternatives, the INTERFERON GAMMA(21,22) for their treatment. In BRAZIL and VENEZUELA are trying to make a vaccine for this pathology.(25,26)
Until a next edition, greetings
Next editions: * SPOROTRICHOSIS (I)
* SPOROTRICHOSIS (II)
* THE BOTULINUM TOXIN
======================================================================
DERMAGIC/EXPRESS(19)
======================================================================
LEISHMANIASIS CUTÁNEA PENTAMIDINA E ITRACONAZOLE
CUTANEOUS LEISHMANIASIS PENTAMIDINE AND ITRACONAZOLE
======================================================================
1.) Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine.
2.) [Treatment of american cutaneous leishmaniasis, with lesions in the mucosa, using
pentamidine isethionate]
3.) Evaluation of pentamidine for the treatment of cutaneous leishmaniasis in Colombia.
4.) Drug delivery system: targeting of pentamidines to specific sites using sugar grafted liposomes.
5.) Diabetes mellitus associated with pentamidine isethionate in diffuse cutaneous
leishmaniasis.
6.) Anti leishmanial therapy--the changing scene [editorial]
7.) Action of pentamidine-bound nanoparticles against Leishmania on an in vivo model.
8.) [Leishmania (Viannia) braziliensis Vianna, 1911 in French Guiana. Clinical, therapeutic and epidemiological considerations in the ninth human diagnosed case]
9.) Treatment of New World cutaneous and mucosal leishmaniases.
10.) [Comparative study of meglumine antimoniate, pentamidine isethionate and aminosidine sulfate in the treatment of primary skin lesions caused by Leishmania (Viannia) braziliensis]
11.) Leishmaniasis: report of 33 cases and a review of the literature [editorial]
12.) Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years.
13.) [Measurement of the volume of the skin ulcer in cutaneous leishmaniasis]
14.) Limited efficacy of injectable aminosidine as single-agent therapy for Colombian cutaneous leishmaniasis.
15.) [Treatment of localized cutaneous leishmaniasis]
16.) Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole.
17.) Cutaneous leishmaniasis in India. Clinical experience with itraconazole (R51 211 Janssen).
18.) Cutaneous leishmaniasis. Treatment with itraconazole.
19.) Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study.
20.) A randomized trial of amphotericin B alone or in combination with itraconazole in the treatment of mucocutaneous leishmaniasis.
21.) Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with
sodium stibogluconate and gamma interferon depends on continued interleukin-12
production.
22.) Successful treatment of cutaneous leishmaniasis using systemic interferon-gamma.
23.) PENTAMIDINE isethionate, the product.
24.) PENTAMIDINE, the product
25.) Vaccine for prophylaxis and immunotherapy, Brazil.
26.) Leishmaniasis: Immunological and clinical aspects and vaccines in Venezuela.
======================================================================
1.) Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine.
======================================================================
Author
Soto J; Buffet P; Grogl M; Berman J
Address
Bogota Military Hospital, Colombia.
Source
Am J Trop Med Hyg, 50(1):107-11 1994 Jan
Abstract
We previously found that 2 mg of pentamidine isethionate/kg, administered every other day
in seven injections, was 95% curative for Colombian cutaneous leishmaniasis. However,
17% of the patients had to prematurely terminate therapy due to drug toxicity and another
30% had mild-to-moderate toxic clinical reactions. In this report, we show that the same
daily dose of drug, 2 mg/kg, but administered in only four every-other-day injections, resulted
in an 84% cure rate in 38 patients. Twenty-one patients (55%) experienced side effects,
three of which were moderate to severe. A higher daily dose of drug, 3 mg/kg, administered
in four every-other-day injections, resulted in a 96% cure rate in 51 evaluable patients.
Thirty-six of the treated patients (64%) experienced side effects, five of which were
moderate to severe. Although hypotension and hypoglycemia were looked for in all patients,
only one patient experienced hypoglycemia and it had normalized by follow-up. We propose
that the regimen of 3 mg of pentamidine/kg every other day in four injections is optimal for
Colombian cutaneous leishmaniasis and competitive with standard Glucantime therapy, in
terms of cure rate, toxicity, length of time the patient has to be under medical supervision, and
cost of drug plus medical attention. We suggest that such a short course of injectable agent
be studied for the treatment of patients with cutaneous leishmaniasis from other endemic
areas.
======================================================================
2.) [Treatment of american cutaneous leishmaniasis, with lesions in the mucosa, using
pentamidine isethionate]
======================================================================
Author
Amato VS; de Paula JG; Imamura R; Amato Neto V; Duarte MI; Boulos MI; Boulos M;
Nicodemo AC; de Mendonc¸a JS
Address
Laborat´orio de Investiga¸c~ao M´edica/Patologia das Mol´estias Infecciosas, Hospital das
Cl´inicas e Departamentos de Doen¸cas Infecciosas e Parasit´arias, Otorrino-Laringologia da
Faculdade de Medicina da Universidade de S~ao Paulo.
Source
Rev Soc Bras Med Trop, 29(5):477-81 1996 Sep-Oct
Abstract
Ten patients with mucosal lesions caused by American tegumental leishmaniasis were
treated with pentamidine isethionate at the dose 4 mg/kg on alternate days by the
intravenous route. The mean posology was 2,140 mg. Healing of the lesions occurred in 9
(90%) of the patients who completed treatment. There was no recurrence during a follow-up
time of 1 to 24 months (mean, 7,7 months). One patient discontinued treatment before
healing of the lesion because be developed diabetes mellitus. In 3 (30%) patients, blood
exams showed increased urea and creatinine levels and leucopenia, which were corrected by
increasing the interval between administrations of the drug. Pentamidine isethionate is
efficient in bringing about cicatrization of the lesions but needs further evaluation in terms of its
value in preventing recurrence.
======================================================================
3.) Evaluation of pentamidine for the treatment of cutaneous leishmaniasis in Colombia.
======================================================================
Author
Soto-Mancipe J; Grogl M; Berman JD
Address
Bogota Military Hospital, Colombia.
Source
Clin Infect Dis, 16(3):417-25 1993 Mar
Abstract
Ninety-two patients in Colombia with cutaneous leishmaniasis were randomly assigned to
groups to be treated with meglumine antimonate (infected control subjects; 10 mg of
antimony/kg intramuscularly, twice a day for 20 days), pentamidine (2 mg/kg every other
day intramuscularly, for a total of seven injections), or itraconazole (200 mg orally, twice a
day for 28 days) or to receive no treatment (negative control subjects). In the group treated
with meglumine antimonate, 21 of 23 patients healed by the first follow-up visit, 1.5 months
after the end of therapy, and did not relapse (91% cure rate). In the pentamidine-treated
group, 23 of 24 patients healed and did not relapse (96%). Four of the 23
pentamidine-treated patients who ultimately were cured had terminated therapy prematurely
(after receiving 4-6 injections) because of hypotension (2 patients), hypoglycemia (1 patient),
or headache and myalgias (1 patient). In a subsequent group of 19 patients who were
administered 2 mg of pentamidine/kg every other day for a total of only four injections, 14
healed without relapse (74% cure rate). The itraconazole-treated group was similar to the
no-treatment control group in terms of the number of patients for whom therapy failed (75%
and 64%, respectively).(ABSTRACT TRUNCATED AT 250 WORDS)
======================================================================
4.) Drug delivery system: targeting of pentamidines to specific sites using sugar grafted liposomes.
======================================================================
Author
Banerjee G; Nandi G; Mahato SB; Pakrashi A; Basu MK
Address
Indian Institute of Chemical Biology, Calcutta, India.
Source
J Antimicrob Chemother, 38(1):145-50 1996 Jul
Abstract
Different sugar-grafted liposomes were prepared and tested against experimental
leishmaniasis in vivo using the classical drug pentamidine isethionate and its methoxy
derivative. Both the drugs, when encapsulated in sugar-grafted liposomes were found to be
more potent in comparison to normal liposome-encapsulated drug or to the free drug.
Moreover, the mannose-grafted liposomes were adjudged to be the best in lowering of
spleen parasite load in comparison with those bearing glucose or galactose. When
encapsulated in mannose-grafted liposomes the therapeutic efficacy of pentamidine
isethionate was found to be better than that of its methoxy derivative, although the latter
seemed to be less toxic than the pentamidine isethionate itself.
======================================================================
5.) Diabetes mellitus associated with pentamidine isethionate in diffuse cutaneous
leishmaniasis.
======================================================================
Author
Costa JM; Moraes MS; Saldanha AC; Barral A; Burattini MN
Address
Departamento de Patologia, Faculdade de Medicina, Universidade Federal do Maranh~ao,
S~ao Luis, MA, Brasil.
Source
Rev Soc Bras Med Trop, 28(4):405-7 1995 Oct-Dec
Abstract
The authors report a case of a male patient from Bacabal, MA with diffuse cutaneous
leishmaniasis (DCL), for at least nine years, with 168 lesions on his body. These were
tumour-like nodules with some ulceration. He used pentavalent antimonial (glucantime) and
an association of gamma interferon plus glucantime with improvement of the lesions but
relapsed later. Recently, pentamidine isethionate (pentacarinat) was given a dosage of
4mg/kg/weight/day on alternate days for 20 applications. After 3 months a similar course of
10 application was given 2 times. Later he developed diabetic signs with weight loss of 10kg,
polydypsia, polyuria and xerostomia. The lower limbs lesions showed signs of activity. Blood
glucose levels normalised and remain like this at moment. Attention is drawn to the fact that
pentamidine isethionate should be used as a therapy option with care, obeying rigorous
laboratory controls including a glucose tolerance test.
Language
======================================================================
6.) Anti leishmanial therapy--the changing scene [editorial]
======================================================================
Author
Bichile LS
Source
J Assoc Physicians India, 42(9):682-3 1994 Sep
======================================================================
7.) Action of pentamidine-bound nanoparticles against Leishmania on an in vivo model.
======================================================================
Author
Fusai T; Deniau M; Durand R; Bories C; Paul M; Rivollet D; Astier A; Houin R
Address
Laboratoire de Parasitologie, Facult´e de M´edecine, Cr´eteil.
Source
Parasite, 1(4):319-24 1994 Dec
Abstract
The efficiency of antileishmanial agents may be enhanced by improving their bioavailability
with a colloidal drug carrier. We have investigated the action of free pentamidine,
compared with pentamidine bound to polymethacrylate nanoparticles, in a rodent model.
BALB/c mice were infected, via the tail vein, with 4 x 10(7) L. major (MON 74)
promastigotes. Twelve days after infection, seven groups of mice were treated respectively
with methylglucamine antimoniate (Glucantime) 5.56 mg/kg i.p. x 5 d., pentamidine bound
nanoparticles (100 microM), unloaded polymethacrylate nanoparticles, unloaded
nanoparticles associated with free pentamidine (100 microM) 0.1 ml i.v. x 3 d and free
pentamidine isethionate (2.28 mg/kg and 0.17 mg/kg i.v. x 3 d.). Twenty-one days post
infection, the mice were sacrificed and the Leishmania load in the liver calculated from the
number of amastigotes/500 liver cells and total liver weight in treated and untreated mice.
Results demonstrated a 77% amastigote reduction in the group treated with targeted
pentamidine relative to the control group. The ratio free pentamidine/bound-pentamidine
was approx. 12.
======================================================================
8.) [Leishmania (Viannia) braziliensis Vianna, 1911 in French Guiana. Clinical, therapeutic and epidemiological considerations in the ninth human diagnosed case]
======================================================================
Author
Raccurt CP; Pradinaud R; Couppie P; Moreau B; Pratlong F; Dedet JP; Cotellon P; Juminer
B; Sainte-Marie D
Address
Laboratoire de biologie polyvalerite, l'universit´e des Antilles, Cayenne.
Source
Bull Soc Pathol Exot, 89(5):341-4 1996
Abstract
The authors report the ninth case of cutaneous Leishmaniasis without mucosal involvement
due to Leishmania (Viannia) braziliensis (isoenzymatic profile related to zymodeme
MON-44) diagnosed in a legionnaire who recently arrived in French Guiana. The skin lesion
as a single ulcerated nodule of the dorsum of the left ringfinger was cured after two courses of
four intramuscular injections of pentamidine isothionate (total posology of
pentamidine-base: 16.6 mg/kg). The transmission occurred during nocturnal trekking in
forest and swamps just behind the coastal belt at Degrad Saramaka (7 km South of Kourou).
In French Guiana, the good level of medical care and the early treatment of the majority of
the cases of Leishmaniasis may explain the rarity of mucosal lesions. Since the clinical
aspect of the lesion is not sufficient to prejudge the identity of the causative species, it is
necessary to perform cultivation of Leishmania for iso-enzymatic identification. The
adaptation of pentamidine doses and long term follow up of patients infected by L. (V.)
braziliensis could be defined more precisely.
======================================================================
9.) Treatment of New World cutaneous and mucosal leishmaniases.
======================================================================
Author
Berman JD
Address
Walter Reed Army Institute of Research, Washington, DC 20307-5100, USA.
Source
Clin Dermatol, 14(5):519-22 1996 Sep-Oct
Abstract
The most extensive investigations of treatment of New World cutaneous leishmaniasis have
been performed against L. panamensis disease in Colombia, and the relative value of
regimens shown there may be instructive for disease from other areas. In Colombia, a
90-95% cure rate was achieved with three different drug regimens: The standard regimen of
pentavalent antimony (20 mg/ kg/day for 20 days parenterally) A short course of
pentamidine (3 mg/kg every other day for four injections intramuscularly The marketed
combination of topical paromomycin (15%)-MBCl (12%) for 10 days, plus antimony (20
mg/kg/day parenterally) for 7 days. My view is that all these regimens could be chosen as
first-line therapy for cutaneous disease in Colombia. The antimony regimen has the advantage
of established use; the disadvantages are cost, requirement for injections each day for 20
days, and considerable morbidity in the last two weeks of therapy. The pentamidine
regimen has the advantage of a short time course; the disadvantages are lack of experience
with this new regimen and frequent, although moderate, morbidity. The combined
topical-parenteral regimen has the advantage of requiring few and nontoxic injections; the
primary disadvantage is that the regimen is novel and its efficacy has not been confirmed. It
would be expected that cases of lesions in other areas caused by L. braziliensis complex
would respond in a similar manner to these regimens. To date, however, only the efficacy of
the standard antimonial regimen has been confirmed. In certain regions of Central America,
other regimens may be effective. Thus, ketoconazole appears to be effective for the more
rapidly self-curing forms of disease (cutaneous disease caused by L. mexicana and L.
panamensis from Central America), and a short course of antimony may be effective against
L. braziliensis in Guatemala.
======================================================================
10.) [Comparative study of meglumine antimoniate, pentamidine isethionate and aminosidine sulfate in the treatment of primary skin lesions caused by Leishmania (Viannia) braziliensis]
======================================================================
Author
Correia D; Mac^edo VO; Carvalho EM; Barral A; Magalh~aes AV; de Abreu MV; Orge
ML; Marsden P
Address
Universidade de Bras´ilia, DF.
Source
Rev Soc Bras Med Trop, 29(5):447-53 1996 Sep-Oct
Abstract
With the aim of comparing the therapeutic efficacy, tolerability and toxicity of meglumine
antimoniate, aminosidine sulphate and pentamidine isethionate, a field study was conducted
on randomized treatment of patients with primary cutaneous leishmaniasis due to
Leishmania (Viannia) braziliensis (L(V)b), in Corte de Pedra, BA, from October 1992 up to
January 1993. Forty six patients were treated and distributed into three groups, two with 15
and one with 16 subjects. All patients were submitted to clinical examination,
histopathological and immunological investigations, as diagnostic criterium. All patients were
treated by intramuscular route. Group 1 received pentamidine 4 mg/kg/every 2 days, for 8
applications; Group 2 received aminosidine 20 mg/kg/day, for 20 days, and Group 3
received meglumine 10 mg Sbv/kg/day, for 20 days. Failure of therapy was defined as
ulceration of the skin lesion four months after treatment. Such failure occurred in five cases as
follows: two cases in patients of group 1 one case in patients of group 2, and two cases in
group 3, after the first year of follow up. In the evaluation after three years we reviewed
fifteen patients, five in each group; except for one in Group 3, all of them were cured.
Statistical significance of the results between the three schedules used was not verified.
======================================================================
11.) Leishmaniasis: report of 33 cases and a review of the literature [editorial]
======================================================================
Author
Bouree P; Belec L
Source
Comp Immunol Microbiol Infect Dis, 16(4):251-65 1993 Oct
Abstract
Leishmaniasis are parasitic diseases in extension. They appear in new foci, because of
important displacements of populations, and they affect immunocompromised patients (under
chemotherapy, transplanted, or HIV infected). Study of 33 cases of leishmaniasis, 22 visceral
and 11 cutaneous, at the H^opital du Kremlin-Bic^etre, France, showed predominant
contamination in Maghreb and in the south of France. In the case of Kala-Azar, fever (18
cases) and hepatosplenomegaly (19 cases) are frequent, and the serodiagnosis and the
search of parasites by myelogram are always positive. In HIV-infected individuals, clinical
signs are similar, but the serodiagnosis is less reliable. Evolution is bad in transplanted patients
who must remain under immunosuppressive drugs. In the case of cutaneous leishmaniasis,
diagnosis is based on local sample, while the serodiagnosis remains negative. Treatment is
sometimes long, necessitating repeated treatments.
Title
[Mucocutaneous leishmaniasis in otorhinolaryngology]
Author
Patuano E; Carrat X; Drouet Y; Barnab´e D; Vincey P; Berthelot B
Address
Service ORL, CHU de Bordeaux.
Source
Ann Otolaryngol Chir Cervicofac, 110(7):415-9 1993
Abstract
The authors present a case of mucocutaneous Leishmaniasis of the nose in a patient
originally from French Guiana and review the characteristics of this parasitic disease and its
importance in the differential diagnosis of lethal midline granuloma. Beyond the exotic
pathology of this case, the review of the literature indicates that with the present extension of
concomitant leishmaniasis and Hids, authentic autochtonal leishmaniasis may be
responsible for otolaryngologic manifestations. The increased prevalence of leishmaniasis
associated with AIDS, as well as the presence of mucosal lesions, seems to be
underestimated.
======================================================================
12.) Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years.
======================================================================
Author
Berman JD
Address
Division of Experimental Therapeutics, Walter Reed Army Institute of Research, Washington,
D.C. 20307-5100, USA.
Source
Clin Infect Dis, 24(4):684-703 1997 Apr
Abstract
The current interest in leishmaniasis stems from the importance of this disease with respect
to travel medicine, veterans of Operation Desert Storm, humanitarian concerns, and infection
with human immunodeficiency virus. Herein, I review aspects of leishmaniasis that are of
practical value to practitioners, including presentation, diagnosis, and chemotherapy; I will
emphasize advances in chemotherapy over the last 10 years. Amphotericin B and its new
lipid formulations are now competitive with pentavalent antimony as primary therapy for
visceral leishmaniasis. Pentamidine, paromomycin, and adjunctive therapy with
interferon-gamma are secondary regimens for the treatment of this condition. High-dose
long-term regimens of antimony have been shown to be highly effective for the treatment of
cutaneous leishmaniasis. Preliminary evidence of efficacy has been observed with short
courses of pentamidine for the treatment of Leishmania braziliensis complex disease and
topical paromomycin/methylbenzethonium chloride for the treatment of Leishmania major
disease.
======================================================================
13.) [Measurement of the volume of the skin ulcer in cutaneous leishmaniasis]
======================================================================
Author
Correia D; Macedo V; Marsden PD
Address
Faculdade de Medicina do Tri^angulo Mineiro, Uberaba, MG.
Source
Rev Soc Bras Med Trop, 29(6):593-8 1996 Nov-Dec
Abstract
Skin ulcers by Leishmania (Viannia) braziliensis are often deep and irregular and are difficult
to measure by just the skin surface transverse and longitudinal diameters. The proposal is to
mould the cavity, after local asepsis with fresh water plus soap, with a gelatinous plastic
which contains silence, potassium alginate, calcium sulphate, magnesium oxide
commercialized under the name of jeltrate (Dentsply Laboratory), by solving 9.5g of jeltrate
in 20ml of fresh water and applying the gel on the ulcer which solidifies in 5 minutes. This
mould is then filled with a self polymerising acrylic and its volume measured either by weight
(by using an analytical balance)-technique 1-or by water displacement by applying
Archimeds'principle-technique 2. We show data in a field trial before and after 20 days
treatment in 20 patients using three different schedules as follows: 7 received pentamidine
isethionate, 7 patients received aminosidine sulphate and 6 received meglumine antimoniate.
The results point out that there was a uniform reduction of ulcer volume occurred during this
period in the three groups, in both technique. Regarding the therapeutic schedules we are
sure that there was a significant statistical difference between the three schedules using the T
Student Test, which showed that aminostdine sulphate produced a better volume reduction of
the ulcer than the other drugs. Serial moulds reflect clinical billing and are a permanent
record. We conclude that the measure of the volume of the skin ulceration can be useful in
the therapeutic evaluation, as a practical and cheap procedure, and may be used in field trials.
======================================================================
14.) Limited efficacy of injectable aminosidine as single-agent therapy for Colombian cutaneous leishmaniasis.
======================================================================
Author
Soto J; Grogl M; Berman J; Olliaro P
Address
Bogota Military Hospital, Colombia.
Source
Trans R Soc Trop Med Hyg, 88(6):695-8 1994 Nov-Dec
Abstract
Ninety military patients with cutaneous leishmaniasis in Colombia were randomly allocated
to 3 treatment regimens of parenteral aminosidine sulphate: (i) 12 mg aminosidine base/kg/d
for 7 d, (ii) 12 mg/kg/d for 14 d, and (iii) 18 mg/kg/d for 14 d. With the 89 evaluable
patients, the cure rates 12 months after the end of treatment were 10%, 45%, and 50%,
respectively. Fifty-eight of the 66 patients who were not cured had lesions that enlarged or
were unchanged by 1.5 months after treatment follow up. The other 8 patients had lesions
that relapsed between 3 and 12 months after therapy. Even in group (iii) the cure rate was
inferior to that (> 90%) with antimony or pentamidine previously reported in this patient
population. This study indicates that parenteral aminosidine alone is less likely to be
successful in the treatment of cutaneous lesihmaniasis than visceral leishmaniasis, for which
a 74% cure rate has been reported. Further trials might consider the combination of
aminosidine with other antileishmanial drugs.
======================================================================
15.) [Treatment of localized cutaneous leishmaniasis]
======================================================================
Author
Buffet P; Caumes E; Gentilini M
Address
H^opital de la Piti´e-Salp^etri`ere, D´epartement de M´edecine tropicale, Maladies
infectieuses, Parasitologie-Mycologie et Sant´e Publique, Paris.
Source
Ann Dermatol Venereol, 121(6-7):503-11 1994
======================================================================
16.) Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole.
======================================================================
SO - Int J Dermatol 1991 Jul;30(7):519-21
AU - al-Fouzan AS; al Saleh QA; Najem NM; Rostom AI
AD - Department of Dermatology, Al-Sabah Hospital, Safat, Kuwait.
MJ - Antifungal Agents [therapeutic use]; Ketoconazole [analogs & derivatives];
PT - CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED TRIAL
AB - Twenty-four patients suffering from single or multiple lesions of cutaneous leishmaniasis were included in this study. Most of the lesions were on the extremities. The patients were randomly divided into two groups. Most of the patients in the first group who were given oral itraconazole for a period of 6-8 weeks showed excellent clinical response. On the other hand, only one patient in the second control group who was given placebo showed good clinical improvement. Systemically administered itraconazole may prove to be a valuable modality for the treatment of cutaneous leishmaniasis.
======================================================================
17.) Cutaneous leishmaniasis in India. Clinical experience with itraconazole (R51 211 Janssen).
======================================================================
SO - Int J Dermatol 1990 Nov;29(9):661-2
AU - Dogra J; Aneja N; Lal BB; Mishra SN
AD - Department of Medicine and Dermatology, S.P. Medical College, Bikaner, India.
PT - CLINICAL TRIAL; JOURNAL ARTICLE; RANDOMIZED CONTROLLED TRIAL
AB - Fifteen patients with cutaneous leishmaniasis were randomly selected for clinical trials with itraconazole. A single daily itraconazole dose of approximately 4 mg per 1 kg of body weight was administered to each patient with breakfast for 6 weeks. Five patients served as controls. On completion of therapy, ten cases in the study group (66.6%) were considered to be cured. No major adverse effects were reported. No significant changes were seen in the controls at the end of the study. The possibility of spontaneous recovery will be discussed in this report even though it is considered to be unlikely. Itraconazole has promising potential in cutaneous leishmaniasis therapy.
======================================================================
18.) Cutaneous leishmaniasis. Treatment with itraconazole.
======================================================================
SO - Arch Dermatol 1989 Nov;125(11):1540-2
AU - Albanese G; Giorgetti P; Santagostino L; Crippa D; Sala G
AD - Department of Dermatology, S. Gerardo Hospital, Monza, Italy.
MJ - Antiprotozoal Agents [therapeutic use]; Ketoconazole [analogs & derivatives]; Leishmaniasis [drug therapy]
MN - Adult; Follow-Up Studies; Ketoconazole [therapeutic use]
MT - Case Report; Human; Male
PT - JOURNAL ARTICLE
AB - The chemotherapy of leishmaniasis is still far from satisfactory. Itraconazole, one of the most recent azole antifungal agents, appears to be well tolerated in man. Two patients with cutaneous leishmaniasis (Leishmania tropica), contracted in Saudi Arabia, were treated orally for 2 months with itraconazole (100 mg/d). Both recovered without side effects or abnormalities in their main biologic parameters.
======================================================================
19.) Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study.
======================================================================
AU: Momeni-AZ; Jalayer-T; Emamjomeh-M; Bashardost-N; Ghassemi-RL; Meghdadi-M; Javadi-A; Aminjavaheri-M
AD: Isfahan University of the Medical Sciences, Iran.
SO: Arch-Dermatol. 1996 Jul; 132(7): 784-6
ISSN: 0003-987X
PY: 1996
LA: ENGLISH
CP: UNITED-STATES
AB: OBJECTIVE: To compare the efficacy of itraconazole with placebo in the treatment of cutaneous leishmaniasis caused by Leishmania major. DESIGN: Double-blind placebo-controlled study. SETTING: Patients were selected from volunteers wit cutaneous leishmaniasis who lived in a hyperendemic area. PATIENTS: One hundred forty patients were randomly selected for this double-blind study. Exclusion criteria were pregnancy, gestation, age younger than 12 years, and duration of disease of more than 4 months. INTERVENTION: Each patient received itraconazole (7 mg/kg per day) or placebo for a 3-week period. The patients were kept under observation for an additional 30-day period. OUTCOME. The study was completed as planned in 131 patients. RESULTS: Complete healing occurred in 59% of the itraconazole group in comparison with 44.3% of the patients who were treated with placebo capsules. No difference was found between the 2 groups with respect to adverse effects. CONCLUSIONS: Although itraconazole has the advantage of being an oral agent that is used in the treatment of cutaneous leishmaniasis, the low response rate in patients receiving itraconazole indicates that itraconazole cannot be used as the single agent in the treatment of patients with cutaneous leishmaniasis caused by L. major.
======================================================================
20.) A randomized trial of amphotericin B alone or in combination with itraconazole in the treatment of mucocutaneous leishmaniasis.
======================================================================
AU: Rodriguez-LV; Dedet-JP; Paredes-V; Mendoza-C; Cardenas-F
AD: Servicio de Dermatologia, Hospital de Clinicas Universitario, Miraflores, La Paz, Bolivia.
SO: Mem-Inst-Oswaldo-Cruz. 1995 Jul-Aug; 90(4): 525-8
ISSN: 0074-0276
PY: 1995
LA: ENGLISH
CP: BRAZIL
AB: A randomized trial of amphotericin B (AB) alone and in combination with oral itraconazole (IZ) is carried out in two groups of 10 mucocutaneous leishmaniasis patients from Bolivia and Peru. AB+IZ combination is no better than AB monotherapy, as far as efficacy and tolerability are concerned. No antagonism was detected.
======================================================================
21.) Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with
sodium stibogluconate and gamma interferon depends on continued interleukin-12
production.
======================================================================
Li J; Sutterwala S; Farrell JP
Department of Pathobiology, University of Pennsylvania, Philadelphia 19104, USA.
Infect Immun (UNITED STATES) Aug 1997 65 (8) p3225-30 ISSN: 0019-9567
Contract/Grant No.: AI-27828--AI--NIAID
Language: ENGLISH
Document Type: JOURNAL ARTICLE
Journal Announcement: 9710
Subfile: INDEX MEDICUS
Treatment of nonhealing forms of human leishmaniasis with antimonial drugs in
combination with gamma interferon (IFN-gamma) may promote healing more effectively
than conventional drug therapy. Although the natures of immune responses in patients
prior to treatment are often unclear, it is generally assumed that such therapy also
promotes a switch from a Th2-type response to a dominant Th1-type response. We have
examined the efficacy of IFN-gamma therapy, in combination with drug therapy, to
promote healing and a Th2-to-Th1 switch in highly susceptible BALB/c mice infected
with Leishmania major. Short-term treatment with the antileishmanial drug sodium
stibogluconate failed to significantly alter the course of disease or the immune
response when it was given during the third and fourth weeks of infection. IFN-gamma
therapy, administered over the same time period, also failed to induce cure or a Th1
dominant response. In contrast, mice treated with a combination of drug and IFN-
gamma therapy resolved their infections and developed Th1-type responses. However,
administration of an antibody to interleukin 12 (IL-12) reversed the therapeutic
effects of therapy with drug plus IFN-gamma, suggesting that IFN-gamma promotes cure
through an IL-12-dependent mechanism. Analysis of mRNA levels within parasitized
lesions suggests that drug treatment plus IFN-gamma treatment, in addition to
reducing parasite numbers, results in reduced levels of IL-4, IL-10, and transforming
growth factor beta transcripts but increased levels of transcripts of the p40 chain
of IL-12 and inducible nitric oxide synthase, which catalyzes the production of
nitric oxide. Together, these results suggest that such immunotherapy may promote
the development of a protective Th1-type response in susceptible mice by a mechanism
which involves both suppression of regulatory cytokines and enhancement of IL-12 and
nitric oxide production.
======================================================================
22.) Successful treatment of cutaneous leishmaniasis using systemic interferon-gamma.
======================================================================
Kolde G; Luger T; Sorg C; Sunderkotter C
Department of Dermatology, and Institute of Experimental Dermatology, University of
Munster, Germany.
Dermatology (SWITZERLAND) 1996 192 (1) p56-60 ISSN: 1018-8665
Language: ENGLISH
Document Type: JOURNAL ARTICLE; REVIEW; REVIEW OF REPORTED CASES
Journal Announcement: 9707
Subfile: INDEX MEDICUS
BACKGROUND: Interferon-gamma (IFN-gamma) is one decisive cytokine of T-helper type
1 (Th1) cells in experimental leishmaniasis. It activates macrophages to kill
intracellular parasites and leads to a decline of less mature macrophages in the
infiltrate. Application of IFN-gamma heals the disease in susceptible mice and has
recently been shown to be of benefit in human disease when given locally or in
combination with antileishmanial drugs. OBJECTIVE: We investigated the clinical and
histological effects of systemic application of IFN-gamma in a case of human
cutaneous leishmaniasis in which an ulcerating lesion endangered the left upper
eyelid of a 4-year-old boy. RESULTS: IFN-gamma (100 mu g/m2 of body surface per day)
was given subcutaneously for a period of 28 days. The well-tolerated treatment
resulted in rapid and complete healing of the lesion without functional impairment.
Histological examination disclosed the formation of dermal granulomas.
Immunohistochemical characterization of the myelomonocytic cells in the lesion before
and after treatment revealed a marked decrease of less-mature macrophages in the
infiltrate. This phenomenon equals observations in healing lesions during naturally
occurring resistance in murine leishmaniasis. CONCLUSION: Systemic monotherapy with
IFN-gamma can be an effective treatment for complicated cases of human cutaneous
leishmaniasis without the side effects sometimes observed with systemic pentavalent
antimony. Its effects on the myelomonocytic infiltrate are similar to the ones
observed during the physiological immune response in natural resistance. (17
References)
======================================================================
23.) PENTAMIDINE isethionate
======================================================================
Trade name(s) PENTACARINAT®
Classification: ANTI-INFECTIVE ANTI-PROTOZOAL
Indication(s): Infections in the early stages of African trypanosomiasis, Pneumocystis carinii pneumonia, and certain forms of
leishmaniasis.
Presentation: 300mg vial.
Storage: Refrigerate - do not freeze. Protect from light.
Reconstitution: WFI. Use 2 to 3mL. Avoid NS for reconstitution.
Stability: Contains no bacteriostat. Stable for 48 hours at 25°C. Discard any unused solution.
Compatible Fluid(s)
G5W; NS† †Infusion only.
Incompatible Fluid(s)
Incompatible Drug(s)
fluconazole, foscarnet
Avoid mixing in solution with any other drugs.
Administration (Mode)
IVI: INTERMITTENT INFUSION
IMI
Intravenous Concentration
Intermittent Infusion: Dilute the required dose in 50 to 250mL.
Intravenous Rate
Intermittent Infusion: Infuse over at least 60 minutes.
Manner of Administration and Dosage Guide
Adult : Pneumocystis carinii pneumonia: 4mg/kg/24 h IVI. Trypanosoma gambiense (early stage): 4mg/kg/24 h daily or
alternate days to a total of 7 to 10 injections, IMI or IVI. Total of 10 doses.
Child : 150mg per m² once daily for 5 days, followed by 100mg per m² once daily for the remainder of the therapy has been
recommended.
Adverse Effect(s)
Hypoglycaemia or hyperglycaemia, hallucinations, hypotension, pancreatitis, cardiac dysrhythmias, tachycardia, leucopoenia,
thrombocytopenia, acute renal failure, hypomagnesaemia, hepatic enzyme elevation, uraemia, anaemia, hyperkalaemia,
hypocalcaemia, nausea and vomiting, dizziness, syncope, flushing, rash, Stevens-Johnson syndrome, taste disturbances,
injection site (pain, discomfort, abscess formation, muscle necrosis), thrombophlebitis
Precaution(s)
Check for hypersensitivity to pentamidine before administration. Avoid direct bolus injection. Patients should remain supine
during administration. The baseline vital signs should be established before the first dose and monitored closely during
administration and regularly until therapy is concluded. Consider dosage modification in the elderly and in patients with renal
dysfunction. Do not administer to pregnant patients unless considered essential. Use with care in patients with malnutrition,
diabetes mellitus, hepatic or renal dysfunction, hypertension and megaloblastic anaemia.
Additional Information
For IMI: WFI. Use 3mL per vial. Avoid intravascular injection. Deep IMI into a large muscle mass (upper outer quadrant of the
buttock or lateral part of the thigh). Before injecting the dose, aspirate to be sure that the needle is not in a blood vessel. If
blood appears, withdraw and inject in another site. Vary the site of injection with repeated doses. Splitting the dose may
minimise local reactions.
Hdx: No Pdx: No data. Sodium: nil Displacement: 0.2mL/300mg
======================================================================
24.) Pentamidine
(WHO essential agent)
======================================================================
Indications: Early West African trypanosomiasis; alternative agent in leishmaniasis (visceral and
certain mucocutaneous forms) and Pneumocystis carinii pneumonia; prophylaxis against P. carinii
pneumonia in AIDS patients (nebulised form).
Pharmacokinetics: Well absorbed from IM injection sites. Blood levels fall rapidly; the drug
accumulates (and is stored) in the liver, spleen and kidneys from where it is slowly released and
excreted via the kidneys for months. Only trace amounts enter the CNS. Minimal absorption occurs
after inhaled use.
Contraindications: Use with caution in diabetics and in patients with renal disease.
Adverse effects: Reversible impairment of renal function; hypo- or hyperglycaemia, hypocalcaemia,
pancreatitis, leucopenia and raised liver enzymes.
Other specific effects - bronchoconstriction with inhalation; severe hypotension with IV
administration; sterile abscess formation at IM injection sites.
Special Prescriber's Points
* Bronchoconstriction with inhaled pentamidine can be minimised by prior administration of inhaled
beta2 agonists.
* Saline solutions should not be used for reconstitution as they cause precipitation. The
recommended diluent is Water for Injection.
* If given IV, infuse slowly over at least 60 minutes with blood pressure monitoring; avoid direct IV
injection.
* Co-trimoxazole has been found more effective as prophylaxis against P. carinii pneumonia in
AIDS patients.
* A specific parenteral preparation is not currently available.
Adult dose: P. carinii pneumonia: Prophylaxis: 300 mg nebulised every 4 weeks (nebuliser must
deliver particles of 1 to 2 micrometers in diameter).
Treatment: IV infusion or IM, 4 mg/kg daily for 14-21 days.
Trypanosomiasis: 4 mg/kg daily or on alternate days for 10 doses.
Visceral leishmaniasis: As for trypanosomiasis.
Cutaneous leishmaniasis: 4 mg/kg once or twice weekly until resolution.
Preparations include:
Pentamidine [INN] [P01CX01]
Pentacarinat® RPR [S4]
pentamidine isethionate
powder for inhalation in solution, 300 mg
======================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS No (19) xx/11/98 DR. JOSE LAPENTA R. DERMATOLOGO
======================================================================
Dr. José Lapenta R.
Maracay, Venezuela
lapentajj@cantv.net
======================================================================
25.) Vaccine for prophylaxis and immunotherapy, Brazil.
======================================================================
Genaro O; de Toledo VP; da Costa CA; Hermeto MV; Afonso LC; Mayrink W
Departmento de Parasitologia, Universidade Federal de Minas Gerais, Brazil.
Clin Dermatol (UNITED STATES) Sep-Oct 1996 14 (5) p503-12
======================================================================
26.) Leishmaniasis: Immunological and clinical aspects and vaccines in Venezuela.
======================================================================
Convit J
Instituto de Biomedicina, Caracas, Venezuela.
Clin Dermatol (UNITED STATES) Sep-Oct 1996 14 (5) p 479-87
=====================================================================
DATA-MÉDICOS/DERMAGIC-EXPRESS No (19) 24/11/98 DR. JOSE LAPENTA R. DERMATÓLOGO
=====================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.024
Tlf: 0414-2976087 - 04127766810


















.png)