REVISTA DERMATOLÓGICA MARZO 2004-2024, EL NIMESULIDE
Hoy 2024, 20 años después, EL NIMESULIDE, APROBADO POR LA FDA para su comercialización, en el año 2002, todavía es vendido en algunos países, que menciono: México, Uruguay, Brasil, y quizá otros.
En Venezuela salió del mercado en el año 2005, en Argentina en el 2009, en la india y Ecuador en 2011, el primero en prohibirlo fue PORTUGAL. En Chile en 2017. En Perú, Paraguay y Panamá nimesulida se retiró definitivamente del mercado en 2007, 2011 y 2015 respectivamente. En Colombia recién en 2024.
Molécula inventada y creada en Estados Unidos, siendo del tipo ANTIINFLAMATORIO NO ESTEROIDEO (AINES), para tratar fiebre (antipirético) y malestar general, cuya característica es ser un inhibidor de la ciclooxigenasa 2 (COX-2).
En varios países NUNCA SE COMERCIALIZÓ, entre ellos el mismo Estados Unidos.
Que tenía esta molécula para que JAMÁS fuera puesta en venta en estos países: Reino Unido, Canadá, Australia, Nueva Zelanda y Japón. ???
Yo conoci una paciente que le extrajeron una muela, le indicaron NIMESULIDE por 5 días para la inflamación y dolor, y al 5to dia MURIO DE UNA NECROSIS HEPÁTICA SEVERA, fui testigo real de lo que es capaz de hacer esta medicina en el organismo.
Molécula FALLIDA que tiene un historial realmente IMPRESIONANTE !
Aqui te dejo este enlace sobre EL NIMESULIDE UNA HISTORIA PARA REFLEXIONAR.
Lee y entenderás el porqué.
DERMAGIC EXPRESS REVISTA MEDICINA Y DERMATOLOGÍA AÑOS 2003-2004 ACTUALIZADO 2024.
Dr. José Lapenta.
ENGLISH
Today, 2024, 20 years later, NIMESULIDE, APPROVED BY THE FDA for marketing in 2002, is still sold in some countries, which I mention: México,Uruguay, Brazil, and perhaps others.
In Venezuela it was taken off the market in 2005, in Argentina in 2009, in India and Ecuador in 2011, the first to ban it was PORTUGAL. In Chile in 2017. In Peru, Paraguay and Panama nimesulide was definitively withdrawn from the market in 2007, 2011 and 2015 respectively. In Colombia, it was not until 2024.
Molecule invented and created in the United States, being of the NON-STEROIDAL ANTI-INFLAMMATORY type (AINES), to treat fever (antipyretic) and general malaise, whose characteristic is to be a cyclooxygenase 2 (COX-2) inhibitor.
In several countries it was NEVER MARKETED, including the United States itself.
What did this molecule have that it was NEVER put on sale in these countries: United Kingdom, Canada, Australia, New Zealand and Japan. ???
I knew a patient who had a tooth extracted, she was prescribed NIMESULIDE for 5 days for inflammation and pain, and on the 5th day SHE DIED FROM SEVERE LIVER NECROSIS, I was a real witness of what this medicine is capable of doing in the body.
A FAILED molecule that has a truly IMPRESSIVE history!
Here I leave you this link about THE NIMESULIDE A STORY TO REFLECT ON.
Read and you will understand why.
DERMAGIC EXPRESS JOURNAL MEDICINA AND DERMATOLOGY YEARS 2003-2004 UPDATED 2024.
Greetings...
Dr. José Lapenta R.
2.) The Origin of the Nimesulide.
3.) Nimesulide not safe, insist doctors.
4.) PIL seeks ban on manufacture, sale of Nimesulide.
5.) INDIA: MEDICAMENTO VINCULADO A MUERTES DISPONIBLE EN EL MERCADO.
8.) Nimesulide-induced fulminant hepatitis.
9.) Efficacy of Nimesulide in Pain Relief after Day Care Surgery.
10.) Hepatitis toxica en gestante por nimesulida.
1.) 2 YEARS OLD BOY DIED FOR PRESUMED BAD MEDICAL PRACTICE in Venezuela Aragua State, Maracay. / (A fallen Angel !!!)
Source: The Century: Maracay Venezuela Aragua State. THURSDAY MARCH 18 2004
Francisco Ronny, a 2 year-old boy's father, I denounce to the
representatives of the press that last Tuesday to the 10.30 30 Pm of
the night died in the Service of Pediatrics of the Central Hospital
of Maracay, his small son because of a presumed bad medical practice
carried out in the population of Villa of Cure.
Ronny who resides in the marked house with the number 93, located in
the Sector 5 Guayabal, in San Francisco of Asis, and that he works
as electrician, I show that their son transfers it to the
Anticancerous of Villa of Cure with a nasal problem, this happened 8
days ago behind.
There, the Dra XXXXX XXXXXXXX prescribed him 2 medications, ONE
WELL-KNOWN AS KLAS (ambroxol-teofilina) and the other
NIMESULIDE, which it should be given them together
with the intention to counterattack the affection that harmed the
boy.
Then the boy's mother, Rosa Aponte, continued to the I tweeted of
the letter the surgeon's recommendations and the Tuesday, in hours
of the morning, KEVIN RONNY APONTE presents an impairment of his
health.
Subsequently it was transferred - she relates - to the Central
Hospital of Maracay, where the medical staff manifested him that
these chemical products should not be given them together, with the
intention of avoiding a strange reaction.
The Physicians ordered him immediately to practice him exams of
toxicology in the Central Hospital of Maracay whose results gave as
positive that the cause of the worsening was the medicins. The death
took place as consequence of a heart arrhythmia....
1.) NIÑO DE 2 AÑOS MURIÓ POR PRESUNTA MALA PRAXIS MÉDICA en Venezuela, Maracay Estado Aragua./ (Un ángel caído !!!)
Fuente: El Siglo: Maracay Venezuela Estado Aragua. JUEVES 18 DE MARZO DE 2004
Francisco Ronny, padre de un niño de 2 años de edad, denuncio a los representantes de la prensa que el pasado martes a las 10.30 P.M. de la noche falleció en el Servicio de Pediatría del Hospital Central de Maracay, su pequeño hijo a causa de una presunta mala práctica médica realizada en la población de Villa de Cura.
Ronny quien reside en la casa marcada con el número 93, ubicada en el Sector 5 Guayabal, en San Francisco de Asís, y que trabaja como electricista, demuestro que su hijo lo traslado al Anticanceroso de Villa de Cura con un problema nasal, esto ocurrió hace 8 días atrás.
Allí, la Dra. XXXXX XXXXXXXX le recetó 2 medicamentos, UNO CONOCIDO COMO KLAS (ambroxol-teofilinato) y el otro NIMESULIDA, los cuales debían serles administrados en conjunto con la intención de contraatacar la afección que perjudicaba al niño.
Luego la madre del niño, Rosa Aponte, continuó al tuitear la carta de las recomendaciones del cirujano, y el martes, en horas de la mañana, KEVIN RONNY APONTE presentó un deterioro de su salud.
Posteriormente fue trasladado -relata- al Hospital Central de Maracay, donde el personal médico le manifestó que estos productos químicos no debían serles administrados en conjunto, con la intención de evitar una reacción extraña.
2.) The Origin of the Nimesulide.
Source: Http://www.essentialdrus.org/
Dr. Chandra M. Gulhati,
Editor, MIMS
e-mail: mims@ndb.vsnl.net.in
Dear All,
While reading all the comments on Nimesulide, it is
noticed that many facts (not opinions) on this drug
are not widely known. Briefly,
1. Nimesulide was discovered by 3M Pharmaceuticals, an
American manufacturer at St. Paul, Minnesota, United
States. Therefore the origin of the molecule is United
States, where it is not approved.
2. As per latest figures, the total world
pharmaceutical market is worth US$ 460 billion out of
which US alone accounts for 50% i.e. US$ 130 billion,
Europe about 25% and rest of the world 25%. Indian's
share is US$ 3.8 billion or less than 1%. Why would an
American company not launch its research product in
its home country? All other products (except
Nimesulid)discovered by 3M Pharmaceuticals are being
marketed in USA. The cost of discovery of a molecule
is US$ 500 to US$ 800 million in USA. This kind of
research expenses can never be recovered without entry
to US market.
3. 3M Riker - the parent company of 3M Pharmaceuticals
- is worth US$ 17 billion and spends over US$ 1
billion every year on research. It sold the molecule
to a small private company in Switzerland called
Helsinn. Helsinn, instead of launching Nimesulide in
Switzerland, licensed the drug to Boehringer in Italy
where it was launched in 1985. In Italy, it is
licensed for use in musculo-skeletal inflammation and
accompanying pain only. Its use in children below 6
years is prohibited.
4. Nimesulide was launched in Switzerland, the home
country of Helsinn, in 1995 i.e. 10 years after Italy
based on the human data from Italy. The Swiss
Government permitted its use in adults only. Its use
in children below 12 years is prohibited.
5. Everyone connected with pharma business around the
world knows that introducing new molecules in USA,
Britian, Australia, Denmark etc. is not very easy
while it is not very difficult in Italy and Brazil.
6. Today Nimesulide is licensed for use in just under
40 countries which also means that it is not permitted
for use in just over 150 countries.
7. In South East Asia (Thailand, Malaysia, Singapore
etc), Nimesulide is permitted to be used only in
adults for "Musculo-skeletal inflamation accompanied
by hyperpyrexia (temperature above 106.7 degree F)"
and no other condition.
8. India is the ONLY country on earth where Nimesulide
drops are marketed for use in neonates and infants.
The only other country where nimsulide is permitted in
children (apart from Italy) is Brazil where it is
prohibited below 3 years.
9. As per Rule 122 (E) of the Drugs & Cosmetics Act
read with Schedule Y, all drugs require mandatory
prior permission from Drugs Controller, India (DCI).
Fresh permission is required if a new formulation of
approved drug is to be launched such as sustained
release etc. In India only two formulations are
approved i.e. 100mg tablet and 50mg/5ml suspension.
The following formulations are being marketed without
DCI permission: 50mg tablets for kids, 25mg/ml drops,
200mg tablets, 400mg tablets, 100mg EF tablets, 100mg
MD tablets. Even when one company obtains permission
for any formulation, another company needs fresh DCI
approval if introducing the same drug within 4 years.
Over 170 companies in India are marketing nimesulide
single ingredient formulations without mandatory DCI
approval.
10. ALL fixed-dose combinations of nimesulide with
other agents such as paracetamol, diclofenac,
tizanidine etc are being marketed without mandatory
DCI approval. This fact has been admitted by DCI in
reply to a starred question in Loksabha on 20-08-2000.
India is the ONLY country on earth where nimesulide
with other agents is being marketed.
11. The retail price of Nimesulide for 10 tablets
ranges between Rs. 14.50 to Rs. 29. It is not the
cheapest "analgesic" or "antipyretic." It is about 200
to 400% more expensive. Besides the cost price of 10
tablets of nimesulide 100mg is Rs. 1.80. As per
ORG-MARG data, no other NSAID has such a huge profit
margin.
12. Several laboratory, animal and human studies in
Canada and USA have shown that Nimesulide is
partially, but not fully, COX-2 Selective. Besides,
Nimesulide inhibits COX-1 much BEFORE and AT MUCH
LESSER SERUM LEVELS. Thus it is not gastro-protective.
This was one of the reasons for aborting an attempt to
get USFDA approval in 1998.
13. All studies submitted to DCI in India by
manufacturers of Nimesulide were sponsored and
financed by Helsinn - many of them actually written by
Helsinn employees.
I will be happy to provide references for above facts
to anyone who needs them.
Dr. Chandra M. Gulhati,
Editor, MIMS
e-mail: mims@ndb.vsnl.net.in
The INDIA-DRUG discussion group is a partnership between
SATELLIFE
(www.healthnet.org), WHO Essential Drugs and Medicines Policy
(www.who.ch), and the Delhi Society for the Promotion of the
Rational Use of Drugs (DSPRUD) in India.
To send a message to india-drug, write to:
india-drug@usa.healthnet.org
To subscribe or unsubscribe, write to:
majordomo@usa.healthnet.org
in the body of the message type: subscribe india-drug OR
unsubscribe india-drug
To contact a person, send a message to:
india-drug-help@usa.healthnet.org

3.) Nimesulide not safe, insist doctors.
Source: Http://www.indiatimes.comKALPANA JAIN
TIMES NEWS NETWORK[ MONDAY, JANUARY 13, 2003 09:59:20 PM ]
DELHI : The `opinion poll' on Nimesulide conducted by the Indian Medical Association calling it `safe' may not end the controversy over the popular fever drug yet. For not only have medical researchers questioned the process but some leading doctors have directly written to the Drug Controller of India to ban its use.
Dr Meharban Singh, a leading pediatrician who is the former head of the pediatrics department at the All India Institute of Medical Sciences, said that it was not correct to issue blanket statements saying the drug is safe. He said he was not part of this IMA opinion poll. ``IMA did not approach me.
I don't know who these 50 people are.'' He said the severe side-effects of the drug have been documented and it needs to be used with caution.Among the doctors who have written to the DCI asking for a ban of the drug are head of pediatrics department at MS Ramaiah Medical College, Bangalore, Dr PP Maiya, past president of National Neonatology Foundation and professor neonatology, KJ Samaiya Medical College, Dr Simen Irani, head of pediatrics department at Calicut Medical College, Calicut, Dr CK Shasidharan, general secretary of the Indian Academy of Pediatrics, Jaipur branch, Dr Susheel Sanghi and president of the Indian Academy of Pediatrics, Jaipur branch, Dr HS Bhasin.
The Nimesulide controversy arose as a result of the government ordering a review of the drug following its ban in several countries. The Drug Controller of India, Dr Ashwini Kumar, who was not available for comment on Monday, had said earlier that the monitoring system in the country was highly inadequate and a ``scientific conclusion''on this top-selling formulation would be difficult.Editor of the Monthly Index of Medical Specialities, Dr CM Gulhati, says with the exception of one or two, no country in the world, allows the use of this drug in children and particularly infants. He said adverse drug reactions are not determined by professional bodies of doctors anywhere in the world.
It is a science by itself and is done through certain mechanisms. Moreover, a word from 50 doctors is not adequate when there are four lakh doctors in the country.Honorary secretary general of the Indian Medical Association, Dr Sanjiv Malik, however, said, the evaluation is as scientific as any evaluation done in the past.
``I am confident we chose a good cross-section of people,'' he said.Optional trim:Researchers point out that the only studies done on the drug have been by the manufacturers themselves. But one of these also showed adverse drug reactions in 261 patients out of a total of 4097. In all the package inserts liver toxicity had not been mentioned as a common adverse drug reaction. On the contrary, they said that ``rarely, a rise in liver enzymes has been reported.''
4.) PIL seeks ban on manufacture, sale of Nimesulide.
Source: Http://www.hindu.com/
By Our Staff Reporter
CHENNAI SEPT. 26. A public interest litigation petition
seeking to ban the manufacture and sale of Nimesulide, a
non-steroidal and anti-inflammatory drug, has been filed in
the Madras High Court.
The First Bench comprising the Chief Justice, B. Subhashan
Reddy, and Justice A. Kulasekaran, admitted the petition filed
by the Tamil Nadu Health Development Forum, and ordered
notices to the Union Health Secretary and the Drugs
Controller-General of India.
The forum secretary and the former Director of the Institute
of Child Health at the Government Children's Hospital here,
C.S. Rex Sargunam, contended that though it was not an
anti-fever drug it can bring down the temperature faster than
other anti-fever drugs like paracetamol.
He said the drug could cause severe sideeffects. ``A person
does not die of fever and joint pain, but dies of liver or
kidney damage caused by the repeated use of Nimesulide''.
According to Dr. Sargunam it was the most expensive
non-steroided anti-inflammatory drug and was not under the
price control regime of the respondent-authorities.
In developed countries such as the United States, Britain,
Canada and Australia, Nimesulide is not approved for use even
for adults, whereas Finland, Spain, Turkey, etc., have banned
the drug, the petition claimed.
Dr. Sargunam prayed for a direction to ban the manufacture and
marketing of Nimesulide.

5.) INDIA: MEDICAMENTO VINCULADO A MUERTES DISPONIBLE EN EL MERCADO.
Source: Http://www.bolentinfarmacos.org/
Sanjay Kumar, BMJ, 2003; 326:70
En la India ha habido un furor mediático por los informes de reacciones adversas a la nimesulida, un fármaco antiinflamatorio no esteroideo, que se ha informado que causa toxicidad hepática. Aunque se aprobó su uso en la India en 1994 para trastornos musculoesqueléticos inflamatorios dolorosos, a menudo se utiliza para aliviar el dolor y la fiebre.
Un fármaco que se sabe que tiene efectos secundarios graves y que ha sido prohibido en algunas partes de Europa todavía está disponible en la India, a pesar de los informes en la prensa sobre varias muertes en el subcontinente entre los niños que lo habían estado tomando.
Author(s): C. Muralikrishna Goud, Syeda Mariya Ghazanfar
Email(s): muralikrishna1789@gmail.com
DOI: 10.52711/2231-5691.2021.00025
Address: C. Muralikrishna Goud*, Syeda Mariya Ghazanfar
Shadan College of Pharmacy, Peerancheru, Himayatsagar Road, Hyderabad, Telangana, India, 500091.
*Corresponding Author
Published In: Volume - 11, Issue - 2, Year - 2021El objetivo del presente trabajo es informar que la nimesulida, un fármaco antiinflamatorio no esteroide, se vende como medicamento de venta libre y se ha prohibido por completo debido a la aparición de hepatitis aguda inducida por nimesulida. El 12 de febrero de 2011, el Ministerio de Salud y Bienestar Familiar de la Unión finalmente decidió suspender el uso pediátrico de la suspensión de nimesulida. A partir del 10 de marzo de 2011, las formulaciones de nimesulida no están indicadas para uso humano en niños menores de 12 años. El 13 de septiembre de 2011, el Tribunal Superior de Madrás revocó una suspensión de la fabricación y venta de los fármacos pediátricos nimesulida y fenilpropanolamina (PPA). Aunque el gobierno de la India ha prohibido el uso pediátrico de nimesulida para la fiebre común y el dolor debido a sus efectos adversos sobre el hígado, su uso por parte de adultos aumenta cada día sin receta médica. El medicamento fue prohibido en 2000 en varios países como Suiza, España, Estados Unidos, etc., mientras que en la India se prohibió en 2011, cuando era demasiado tarde para prohibirlo y todavía estaba disponible en la India para uso adulto a pesar de su hepatotoxicidad y posibles interacciones farmacológicas.
En la India ha habido un furor mediático por los informes de reacciones adversas a la nimesulida, un fármaco antiinflamatorio no esteroideo, que se ha informado que causa toxicidad hepática. Aunque se aprobó su uso en la India en 1994 para trastornos musculoesqueléticos inflamatorios dolorosos, a menudo se utiliza para aliviar el dolor y la fiebre.
6.) [Bleeding gastric ulcers and acute hepatitis: 2
simultaneous adverse reactions due to nimesulide in a
case]
Rev Med Chil. 2000 Dec;128(12):1349-53.[Article in Spanish]
Tejos S, Torrejon N, Reyes H, Meneses M.
Servicios de Medicina y de Anatomia Patologica, Hospital del Salvador, Departamento de Medicina (Campus Oriente), Facultad de Medicina, Universidad de Chile, Santiago, Chile. stejos@alfared.cl
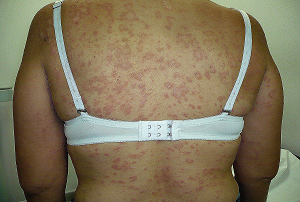
A 66 year-old obese woman with arthrosis, self-medicated with oral nimesulide, 200 mg daily. After 6 weeks she developed nausea, jaundice and dark urine. Two weeks later she had recurrent hematemesis and was hospitalized. Besides obesity and anemia her physical examination was unremarkable. An upper GI endoscopy revealed 3 acute gastric ulcers and a 4th one in the pyloric channel. Abdominal ultrasonogram showed a slightly enlarged liver with diffuse reduction in ecogenicity; the gallbladder and biliary tract were normal. Blood tests demonstrated a conjugated hyperbilirubinemia (maximal total value: 18.4 mg/dl), ALAT 960 U/l, ASAT 850 U/l, GGT 420 U/l, alkaline phosphatases mildly elevated, pro-time 49% and albumin 2.7 mg/dl. Serum markers for hepatitis A, B and C viruses were negative. ANA, AMA, anti-SmA, were negative. Ceruloplasmin was normal. A liver biopsy showed bridging necrosis and other signs of acute toxic liver damage. Gastric ulcers healed after conventional treatment and hepatitis subsided after 2 months leaving no signs of chronic liver damage. The diagnosis of toxic hepatitis due to nimesulide was supported by the time-course of drug usage, sex, age, absence of other causes of liver disease, a compatible liver biopsy and the improvement after drug withdrawal. Peptic ulcers or toxic hepatitis have been previously described as independent adverse reactions in patients taking nimesulide or other NSAIDs but their simultaneous occurrence in a single patient is a unique event that deserves to be reported.
7.) Nimesulide-induced severe hemolytic anemia and
acute liver failure leading to liver transplantation.
Scand J Gastroenterol. 2002 Nov;37(11):1341-3.
Rodrigo L, de Francisco R, Perez-Pariente JM, Cadahia V, Tojo R,
Rodriguez M, Lucena MI, Andrade RJ.
Gastroenterology Service, Hospital Central de Asturias, Oviedo,
Spain.
We present the case of a 63-year-old woman who had undergone 7
months of treatment with Nimesulide (100 mg/b.i.d.) for
symptomatic osteoarthritis. The patient was admitted to our unit
with a clinical picture of progressive jaundice over 3 weeks.
Clinical and analytical studies revealed acute liver failure,
this being confirmed by liver biopsy, which showed submassive
necrosis. Serological tests for different viral agents causing
hepatitis were all negative. In addition, she presented a
picture of severe haemolytic anaemia resistant to several
treatments and needed multiple transfusions. Twenty-three days
after admission, the patient presented hepatic encephalopathy
and received an orthotopic liver transplant on day 25. The
evolution after transplantation was good and the patient
continues in good health with no evidence of haemolysis almost 2
years later. Liver toxicity due to Nimesulide is well known, but
to our knowledge the occurrence of haemolytic anaemia has not
been related to this drug previously. For these reasons,
Nimesulide has been restricted or removed from the market in
several countries in recent months.
![]()
8.) Nimesulide-induced fulminant hepatitis.
Turk J Gastroenterol. 2003 Sep;14(3):208-10.
Ozgur O, Hacihasanoglu A, Karti SS, Ovali E.
Division of Gastroenterology, School of Medicine, Karadeniz Technical University, Trabzon, Turkey. ozgur@ktu.edu.tr
An 18-year-old boy was brought to the hospital with jaundice, confusion, abdominal discomfort and distension. He had a history of oral intake of nimesulide for three days. Clinical and laboratory findings were compatible with fulminant hepatitis. Exclusion of other causes of liver injury strongly favored drug-induced toxicity. All of the signs, symptoms and laboratory abnormalities returned to normal after cessation of the nimesulide and supportive treatment, and he was discharged on the 15th day after admission. This case differs from the other cases in the literature regarding the time of onset, and indicates that nimesulide may induce fulminant hepatitis in the first few days of administration. Therefore, patients receiving nimesulide should be frequently monitored with serial serum transaminases, beginning from the first week of intake.
9.) Efficacy of Nimesulide in Pain Relief after Day Care Surgery
Indian Pediatrics 2002; 39:886-867
I have certain queries and concerns regarding the article(1) published in Indian Pediatrics. How soon post-operatively was the first dose of the drug given and were the drugs were given round the clock or only on a need basis? The type of anesthesia used is not mentioned and this would obviously affect the appearance and extent of pain during the first few hours after surgery. The authors have mentioned that the efficacy of treatment was evaluated by physiological responses, wound hyperthermia and pain behavior. Since this study was done on day care patients it is assumed that they were sent home after a few hours. Who assessed these parameters at home and were they incorporated into the final scores? As we know, facial expression scores are ambiguous at the best and difficult to appreciate in a crying child. The revised pain scale, which has been validated for children between 4-16 years and is based on how a child feels rather than how the face looks, would have been more appropriate(2). Since the study was blinded, I would also like to know who provided the paracetamol to the control group (sponsors or investigators) and why randomization was not done, as the type of surgery would also have affected the degree of pain. Lastly, though there is no information on any side effects in the study population, > 50% were lost to follow-up and hence it is difficult to comment on the safety of the drug. Finally and most importantly, we know that there is controvery regarding Nimesulide which is still not approved by USFDA, Canada, UK, Australia, New Zealand and the Scandinavian countries. It is pertinent to mention that countries like Portugal have withdrawn the drug for Pediatric use since 1999. Developing countries like Sri Lanka have still not approved the drug mainly because they prefer to go along with the USFDA. The study was funded by Panacea Biotec which is a leading manufacturer of the drug in India. Any new drug should be assessed with caution. As responsible members of the medical fraternity in India, where new drugs are readily approved, we need to be careful not to allow competing interests to potentially affect our scientific evaluation(3).
10.) Hepatitis tóxica en gestante por nimesulida.
Hepatitis tóxica en gestante por nimesulida
SOURCE:https://www.elsevier.es/es-revista-gastroenterologia-hepatologia-14-articulo-hepatitis-toxica-gestante-por-nimesulida-12850
J. PEREZ-MORENO, R.M. LLERENA GUERRERO M. PUERTAS MONTENEGRO M.J.
JIMeNEZ ARJONA
Servicio de Aparato Digestivo. Hospital Universitario de
Puerto Real. Puerto Real. Cadiz.
Residente de Medicina
Familiar y Comunitaria. Hospital Universitario de Puerto Real.
Puerto Real. Cadiz.
Servicio de Aparato Digestivo. Hospital
Universitario de Puerto Real. Puerto Real. Cadiz.
Nimesulide-induced toxic hepatitis in pregnancy
Sr. Director: Es bien conocida la potencial capacidad de provocar daño hepático de los antiinflamatorios no esteroides (AINE) en grado variable, desde pequeñas elevaciones de las enzimas hepáticas hasta cuadros graves de hepatonecrosis aguda y/o colestasis1. La incidencia comunicada resulta globalmente baja si se tiene en cuenta la enorme difusión de prescripción de este grupo terapéutico 2. La nimesulida es un AINE comercializado en España en 1997, cuya molécula deriva del grupo de la sulfonanilida, con una acción inhibidora selectiva de la enzima ciclooxigenasa 2 (COX-2), lo que le proporciona un potente efecto antiinflamatorio, analgésico y antipirético 3. Su perfil farmacodinámico reduce los efectos secundarios gastrointestinales, y aunque se había observado la posibilidad de provocar leves alteraciones analíticas hepáticas, no es hasta 1998 cuando se implica en fenómenos de hepatotoxicidad con la comunicación de seis casos de hepatitis aguda bien documentados 4. Recientemente, se han publicado los tres primeros casos acontecidos en España 5; no obstante, queremos reseñar la comunicación de una serie de 12 casos de toxicidad hepática por nimesulida, ocurridos en Argentina entre 1986 y 1996, al XXII Congreso Nacional de la Asociación Española para el Estudio del Hígado de 19976.
Caso clínico. Mujer de 22 años, gestante de 5 semanas, con antecedentes de asma extrínseca por alergia a ácaros y pólenes, y hepatitis A. Niega consumo de alcohol. Tres años antes había sido estudiada en el servicio de hematología por macrocitosis sin causa aparente, recibiendo tratamiento con ácido fólico. Ingresa por cuadro de malestar abdominal, náuseas y vómitos bilio alimenticios y sensación de malestar general, instaurándose a las 24 h tinte amarillento de piel y orinas colúricas. En la anamnesis se recogió el antecedente de ingesta de nimesulida, 100 mg/12 h, durante 5 días, prescrita por un esguince de tobillo, 4 semanas antes. La exploración física no fue relevante salvo la constatación de ictericia cutáneo mucosa sin megalias ni dolor en la palpación abdominal. El servicio de ginecología confirmó su embarazo tras la exploración, test enzimático de detección de HCG en orina y hallazgos ecográficos. La analítica evidenció normalidad hematimetría y de la coagulación, con elevación de AST: 298 U/l; ALT: 635 U/l; GGT: 255 U/l; FA: 309 U/l, y bilirrubina total: 4,7 mg/dl, a expensas de la fracción directa. El resto de parámetros bioquímicos, incluyendo estudio férrico, ceruloplasmina y alfa-1-antitripsina, fueron normales, así como los anticuerpos antinucleares, antimitocondriales, antimúsculo liso y anti-LMK. Los marcadores virales VHB y VHC, incluido ARN por PCR, resultaron también negativos, del mismo modo que el estudio serológico frente a CMV, VEB y VHS I y II. La ecografía abdominal no presentó hallazgos patológicos. La evolución clínica fue favorable, normalizándose transaminasas y bilirrubina en 10 días y persistiendo una GGT de 141 U/l que resultó normal al mes del ingreso. El embarazo de la paciente evolucionó sin incidencias clínicas relevantes, llegando a término con un posterior parto eutócico.
Durante el embarazo se pueden reconocer distintos tipos de hepatopatías, algunas directamente relacionadas con la gestación, otras existentes previamente y otras que aparecen de forma intercurrente, como hepatitis virales o tóxicas, colecistitis y colelitiasis o síndrome de Budd-Chiari. En nuestro caso se estableció el diagnóstico diferencial con la hiperémesis gravídica, entidad que aparece en el primer trimestre del embarazo, pero se descartó dada la falta de correlación clínica y analítica, no considerándose otros cuadros propios de la gestación, tales como la colestasis intrahepática, la esteatosis aguda, la hepatopatía asociada a preeclampsia y eclampsia o el síndrome. HELLP, por ser propios de estadios más avanzados de la gestación 7. El control del embarazo de la paciente que nos ocupa se realizó sin que apareciesen nuevas manifestaciones clínicas o analíticas de afectación hepática.
La incidencia de hepatotoxicidad farmacológica durante el embarazo no parece diferir de la observada en el resto de la población, aunque en teoría podría ser más frecuente o grave ya que está disminuido el aclaramiento hepático y hay inhibición de la función excretora8.
Descartada la relación con la gestación, así como otras posibles causas de hepatitis aguda, tales como la etiología viral, alcohólica, autoinmune, metabólica y patología biliar, la asociación con nimesulida se basó en la relación temporal entre la ingesta del fármaco y la aparición de la hepatitis, la especificidad del cuadro con casos similares de hepatotoxicidad descritos en la bibliografía y la remisión del proceso con la interrupción del fármaco, no considerándose su readministración.
En las dos series publicadas 4,5, los casos incidieron fundamentalmente en mujeres con una edad media de 60 años (rango: 39-81), predominando un cuadro de hepatitis aguda colestásica que remitió entre 8 y 120 días después de su aparición. En los dos únicos casos que afectaron a varones, se presentó un daño colestásico intrahepático grave, habiéndose apuntado la posibilidad de diferentes patrones lesivos asociados al sexo4.
En cuanto al mecanismo lesional, y como ocurre en la mayoría de las reacciones hepáticas adversas por AINE 9, parece corresponder a un mecanismo idiosincrásico de tipo metabólico dependiente del paciente, que aparece tras un período variable de exposición al tóxico por la existencia de vías metabólicas aberrantes 10. No obstante también se han observado signos de hipersensibilidad en dos de los casos descritos 4,5 en probable relación con la molécula sulfonanilida de la nimesulida, que provocaría una reacción alérgica similar a la dapsona o la sulfapiridina 4.
Con la comunicación de nuestra experiencia con este nuevo antiinflamatorio, y a pesar de que se trate de una reacción adversa muy infrecuente, consideramos que una monitorización adecuada permitiría predecir y objetivar la hepatotoxicidad de fármacos de reciente introducción en el arsenal terapéutico.
Bibliografía
[1] Manoukian AV, Carson JL..
Nonsteroidal anti-inflammatory drug-induced hepatic disorders. Incidence and prevention..
Drug Saf, 15 (1996), pp. 64-71
Medline
[2] García Rodríguez LA, Williams R, Derby LE, Dean AD, Jick H..
Acute liver injury associated with nonsteroidal antiinflammatory drugs and the role of risk factors..
Arch Intern Med, 154 (1994), pp. 311-316
Medline
[3] Davis R, Brogden RN..
Nimesulide. An update of itspharmacodynamic and pharmacokinetic properties, and therapeutic efficacy..
Drugs, 48 (1994), pp. 431-454
Medline
[4]Van Steenbergen W, Peeters P, De Bondt J, Staessen D, Büscher H, Laporta T et al..
Nimesulide-induced acute hepatitis: evidence from six cases..
J Hepatol, 29 (1998), pp. 135-141
Medline [5] Romero-Gómez M, Nevado Santos M, Fobelo MJ, Castro Fernández M..
Hepatitis aguda por nimesulida: descripción de tres casos..
Med Clin (Barc), 113 (1999), pp. 357-358
[6] Bessone F, Fay F, Fay O, Vorobioff J, Passamonti M, Tanno H..
Toxicidad hepática por nimesulida. Presentación de 12 casos..
Gastroenterol Hepatol, 20(Supl1) (1997), pp. 35
[7] Muñoz R, Castellano G..
Hepatopatía y gestación..
Rev Gastroenterol Interhospitalaria, 1 (1997), pp. 38-44
[8] Knox TA, Olans LB..
Liver disease in pregnancy..
N Engl J Med, 335 (1996), pp. 569-576
http://dx.doi.org/10.1056/NEJM199608223350807 | Medline
[9] Boelsterli UA, Zimmerman HJ, Kretz-Romme A..
Idisyncratic liver toxicity of nonsteroidal antiinflammatory drugs: molecular mechanisms and pathology..
Crit Rev Toxicol, 25 (1995), pp. 207-235
http://dx.doi.org/10.3109/10408449509089888 | Medline
[10]Banks AT, Zimmerman HJ, Ishak KG, Harter JG..
Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the food and drug administration as adverse reactions..
Hepatology, 22 (1995), pp. 820-827 Medline
=============================================================
DATA-MEDICOS /DERMAGIC-EXPRESS /MARCH JOURNAL 2004 / DR. JOSE LAPENTA R. DERMATOLOGIST
============================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.0024
Tlf: 0414-2976087 - 04127766810



















.png)