REVISTA DERMATOLÓGICA SEPTIEMBRE 2004, VIOXX- ROFECOXIB
En el año 2001 hice una publicación, haciendo una ADVERTENCIA del PELIGRO, o RIESGO de UTILIZAR, las moléculas inhibidoras de la CICLOOXIGENASA 2 (COX-2), refiriéndome concretamente al NIMESULIDE, posteriormente lo hice sobre el VALDECOXIB (BEXTRA), y el ROFECOXIB, (VIOXX), del laboratorio MERCK.
Destacando sus efectos adversos sobre todo cardíacos. La MOLÉCULA ROFECOXIB BAJO EL NOMBRE DE vioxx, fue lanzada al mercado en MAYO de 1999, aprobada por LA FDA.
Duró 5 años en el mercado produciendo unas ganancias MILLONARIAS al laboratorio, pero también dejó una estela de muertes por eventos cardiovasculares IMPRESIONANTE.
El evento que terminó de explotar esta molécula fue la MUERTE de un atleta de élite de los Estados Unidos, la esposa demandó a MERCK y ganó la demanda por 300 millones de dólares, pero eso no le devolvió la vida a su esposo.
El 30 de Septiembre del 2004 Merck decide sacar del mercado AL Vioxx, por sus deletéreos efectos cardiacos.
Hoy dia 2024, ROFECOXIB, (Vioxx), VALDECOXIB (Bextra), AULIN (Nimesulide), TODAS ella inhibidoras de la ciclooxigenasa dos (COX-2) ya están fuera del mercado. EL NIMESULIDE apenas se vende en algunos países de Sudamérica, pero está prácticamente PROHIBIDO en el resto del mundo.
Quedan en el mercado todavía estas moléculas COX-2: ETORICOXIB (Arcoxia), CELECOXIB (Celebrex), y PARECOXIB (Dynastat).
Aqui te dejo 12 referencia que ratifican lo antes dicho.
Saludos,,,
Dr. José Lapenta.
ENGLISH
In 2001 I made a publication, making a WARNING of the DANGER, or RISK OF USING, the molecules that inhibit CYCLOOXYGENASE 2 (COX-2), referring specifically to NIMESULIDE, later I did it about VALDECOXIB (BEXTRA), and ROFECOXIB, (VIOXX), from the MERCK laboratory.
Highlighting its adverse effects, especially cardiac. The ROFECOXIB MOLECULE UNDER THE NAME OF VIOXX, was launched on the market in MAY 1999, approved by THE FDA.
It lasted 5 years on the market, producing MILLION DOLLAR profits for the laboratory, but it also left an IMPRESSIVE trail of deaths from cardiovascular events.
The event that ended up exploding this molecule was the DEATH of an elite athlete from the United States, the wife sued MERCK and won the lawsuit for 300 million dollars, but that did not bring her husband back to life.
On September 30, 2004, Merck decided to take Vioxx off the market, due to its deleterious cardiac effects.
Today, in 2024, ROFECOXIB, (Vioxx), VALDECOXIB (Bextra), AULIN (Nimesulide), ALL of them inhibitors of cyclooxygenase 2 (COX-2) are already off the market. NIMESULIDE is barely sold in some countries in South America, but it is practically BANNED in the rest of the world.
These COX-2 molecules are still on the market: ETORICOXIB (Arcoxia), CELECOXIB (Celebrex), and PARECOXIB (Dynastat).
Here are 12 references that confirm what was said above.
Greetings...
Dr. José Lapenta R.
=====================================================================
REFERENCIAS BIBLIOGRÁFICAS / BIBLIOGRAPHICAL REFERENCES
=====================================================================
A.- El retirado Vioxx provocó entre 88.000 y 140.000 casos de problemas de corazón en EE.UU.
B.- Merck retira Vioxx del mercado y sus acciones se desploman.
C.- Merck retira «Vioxx», un fármaco utilizado por 277.000 españoles,
al aumentar el riesgo cardiovascular
D.- Merck se declara culpable de publicidad engañosa.
E.- Merck se enfrenta a miles de demandas millonarias por el fármaco
«Vioxx»
1.) Merck Announces Voluntary Worldwide Withdrawal of VIOXX.
2.) Merck retira ‘Vioxx’ del mercado por incremento del riesgo
cardiovascular en uso prolongado.
3.) Merck retira Vioxx por riesgos cardiacos.
4.) Merck halts Vioxx sales.
5.) Merck Halts Vioxx Sales on Health Threats.
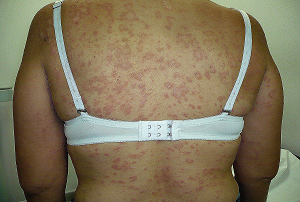
6.) A Case of Rofecoxib-Associated Stevens-Johnson Syndrome With Corneal
and Conjunctival Changes.
7.) Pfizer Affirms Celebrex Safety ???????????
=======================================================
1.) Merck Announces Voluntary Worldwide Withdrawal of VIOXX
Source: Http://healthorbit.ca/
journal@lists.healthorbit.ca <journal@lists.healthorbit.ca>
WHITEHOUSE STATION, N.J., Sept. 30, 2004 - Merck &. Co., Inc. today
announced a voluntary worldwide withdrawal of VIOXX (rofecoxib), its
arthritis
and acute pain medication. The company's decision, which is effective
immediately,
is based on new, three-year data from a prospective, randomized, placebo-
controlled clinical trial, the APPROVe (Adenomatous Polyp Prevention on
VIOXX) trial.
2.) Merck retira ‘Vioxx’ del mercado por incremento del riesgo cardiovascular en uso prolongado.
Source: www.correofarmaceutico.com/
La multinacional Merck, que opera como MSD en Europa, retiró ayer del
mercado su fármaco superventas Vioxx (rofecoxib), inhibidor selectivo de
la
COX-2, indicado para el tratamiento sintomático de la artritis reumatoide
y de la
artrosis y comercializado como Ceoxx en España para el tratamiento
sintomático
del dolor agudo a corto plazo, por su riesgo incrementado de accidente
cardiovascular grave en tratamientos prolongados.
Valvanera Valero
Los nuevos datos del perfil de seguridad del Vioxx se han obtenido en un
ensayo
clínico de rofecoxib frente a placebo para estudiar si previene la
recurrencia de po
polipos colorrectales en pacientes con historia de adenomas colorrectales.
A partir de
los 18 meses de toma diaria de 25 mg de rofecoxib, los pacientes
presentaron un
numero de eventos cardiovasculares superior a placebo (45 frente a 25).
La Agencia Española del Medicamento y Productos Sanitarios (Aemps)
recuerda
en una nota informativa de comunicación de riesgos de medicamentos para
los
profesionales sanitarios que “la seguridad cardiovascular de rofecoxib y
de otros
coxibs ha sido revisada en repetidas ocasiones por las agencias
reguladoras
nacionales desde la publicación del ensayo clínico Vigor, en el que se
observo que
rofecoxib, a dosis de 50 mg (entre 2 y 4 veces la recomendada) se asocia a
un
incremento de riesgo de infarto agudo de miocardio comparado con
naproxeno,
AINE no selectivo”. Entonces, dice la Aemps, “aunque no pudo resolverse si
este
resultado se debía a un incremento de riesgo de rofecoxib o a un efecto
protector
de naproxeno, se procedió a modificar la información del medicamento
dirigida a
los profesionales y los pacientes para advertir de estos resultados”.
Posteriormente,
según la agencia, se han publicado varios estudios donde se observaba un
incremento de riesgo pero solo a dosis superiores a 25 mg.
La AEMPS insiste en que “los datos se refieren unicamente a rofecoxib y
que no
pueden generalizarse a otros inhibidores selectivos de la COX-2”. Y
aconseja que
aunque los riesgos detectados solo ocurren tras un tratamiento prolongado
(mas de
1 año) con Vioxx, se recomienda la suspensión del mismo puesto que es
facilmente
sustituible por tratamientos alternativos sobre los cuales el medico de
familia podrá
determinar la alternativa mas adecuada a las necesidades de los
pacientes.
Vioxx facturo en 2003 2.300 millones de euros en el mundo y desde enero de
2004, 20 millones de euros en España. En los últimos doce meses se han
tratado
en España 277.000 pacientes con el fármaco, aunque no necesariamente en
uso
crónico. Actualmente Sanidad estima que podra haber entre 70.000 y 100.000
pacientes en tratamiento con este fármaco, que requiere como otros coxibs
visado
de inspección desde julio de 2002 en España. Ceoxx, sin embargo, no
esta
cubierto por la seguridad social.
3.) Merck retira Vioxx por riesgos cardiacos.
Source: http://noticias.canalrcn.com/
Nueva York, EE.UU.(RCN) - La farmacéutica Merck hizo este anuncio tras
los
resultados de un estudio que confirmo un mayor riesgo de problemas
cardiacos y
de otro tipo en los pacientes que reciben este tratamiento.
Ante la divulgación de la noticia, las acciones de la empresa cayeron casi
el 27 por
ciento en el mercado de Nueva York, donde media hora después de comenzar
la
sesión se cotizaban en torno a los 33 dólares.
En un comunicado entregado a los medios de comunicación, la empresa indico
que
el estudio, llevado a cabo durante tres años, revelo un mayor riesgo de
sufrir
problemas cardiovasculares, como "un ataque cardíaco o un derrame
cerebral",
después de 18 meses de tratamiento en los pacientes que tomaron Vioxx que
en
los que tomaron placebos.
4.) Merck halts Vioxx sales.
By Rita Rubin, USA TODAY
Source: http://www.usatoday.com/
The maker of blockbuster pain reliever Vioxx said Thursday that it is
voluntarily
withdrawing the drug because of new data showing it increases the risk of
heart
attacks and strokes.
By Mike Derer, AP
This is one of the largest-ever withdrawals of a drug, says the Food and
Drug
Administration's Steven Galson. Drugmaker Merck (MRK) says 84 million
people
worldwide have taken the heavily promoted drug, available in the USA since
May
1999. About 2 million Americans are on Vioxx, Galson says. "We have been
concerned and aware of the potential for cardiovascular effects for the
last few
years," he says. "This is not a total surprise."
The news hit Merck's stock hard. It fell 27% to close at $33, wiping out
$27 billion
in market value.
In August, an FDA-funded analysis involving Kaiser Permanente patients
found
more heart attacks and sudden cardiac deaths among people taking Vioxx
than
among those on Celebrex or on conventional nonsteroidal anti-inflammatory
drugs
(NSAIDs) such as ibuprofen and aspirin.
In November 2000, Merck published a study in The New England Journal of
Medicine that found a higher rate of heart attacks in patients assigned to
Vioxx than
those assigned to naproxen (an NSAID sold as Aleve). Because of that
finding, the
FDA in April 2002 required a new warning on the Vioxx label.
Vioxx, Celebrex and Bextra are the three so-called COX-2 inhibitors on the
U.S.
market. Drugmakers say they treat arthritis pain but are less irritating
to the
gastrointestinal tract than traditional NSAIDs. At least two other COX-2
inhibitors
— including Merck's Arcoxia, already sold in 47 other countries — are in
the
pipeline.
"It is important to note that the results of clinical studies with one
drug in a given
class are not necessarily applicable to others in a class," says Peter
Kim, president
of Merck Research Laboratories.
The implications of the Vioxx withdrawal for other COX-2 inhibitors aren't
yet
clear. Merck says the new data — from a three-year study comparing Vioxx
with a
placebo in 2,600 patients with colon polyps — showed a higher risk of
heart
attacks and stroke only after 18 months of use. None of the other COX-2
drugs
have data for longer than a year, Galson says.
"It's too early for me to say right now how we're going to change our
requirements
for the future, but, obviously, we're going to be more interested in
long-term data,"
he says.
5.) Merck Halts Vioxx Sales on Health Threats
Source: http://www.abcnews.go.com/
Merck Pulls Vioxx Arthritis Drug From Market on Heart Attack, Stroke
Concerns; Stock Plunges
The Associated Press
TRENTON, N.J. Sept. 30, 2004 — Merck & Co. is pulling its blockbuster
Vioxx
from the market after new data found the arthritis drug doubled the risk
of heart
attacks and strokes. Merck's stock plunged almost 27 percent as the
pharmaceutical giant said the recall will hurt its earnings.
Merck said Thursday the clinical trial data showed an increased risk of
heart attack
and other cardiovascular complications 18 months after patients started
taking
Vioxx, which also is prescribed for acute pain and disorders such as
carpal tunnel
syndrome.
The three-year study aimed at showing that Vioxx could prevent the
recurrence of
polyps, which can turn cancerous, in the colon and rectum was stopped
after
Merck discovered participants had double the risk of a heart attack
compared to
those taking a placebo.
By Thursday afternoon, at least one plaintiffs' attorney announced plans
for a class
-action lawsuit against Merck. Another claimed to represent 58 patients
around the
country allegedly harmed by Vioxx, including people who suffered a heart
attack,
stroke, internal bleeding or kidney failure.
"It's a disaster for Merck, coming at the worst time," said independent
health care
analyst Hemant Shah of HKS & Co. in Warren, N.J.
About 2 million people worldwide use Vioxx, Merck said, and 84 million
have
taken it since it came on the market with great fanfare in 1999. It is one
of Merck's
most important drugs, with $1.8 billion in U.S. sales in 2003 and global
sales of $2
.5 billion 11 percent of the company's $22.49 billion in revenue that
year.
But Vioxx sales dipped 18 percent in the second quarter of this year to
$653
million, partly due to increasing concerns about an elevated risk of heart
complications.
Medical experts advised patients Thursday to stop taking Vioxx and consult
their
doctor about alternatives.
Merck said the recall will slash about 50 cents to 60 cents a share from
its earnings
for the rest of this year. That includes foregone sales, writeoffs of
inventory held by
Merck, customer returns of product previously sold and other costs of the
pullback. Merck expects foregone fourth quarter sales of Vioxx of $700
million to
$750 million alone.
Merck, based in Whitehouse Station, N.J., had previously been expecting
2004
earnings per share of $3.11 to $3.17.
"We're taking this action because we believe it best serves the interest
of patients,"
Ray V. Gilmartin, Merck's chairman, president and chief executive, said in
a
statement.
Shares in Merck, the world's third-biggest drug maker, plunged $12.07,
nearly 27
percent, to close at $33.00 on the New York Stock Exchange. That wiped out
$
28 billion in market value. More than 140 million shares were traded,
compared to
a daily average below 10 million.
Shah said for Merck, the Vioxx withdrawal comes "at a time when they
really need
to get ready for expiration" of its patent for Zocor, the cholesterol
treatment that is
the company's top-selling drug.
Zocor loses patent protection early in 2006 and sales are expected to
plunge
against generic competition. In an effort to replace those revenues, Merck
recently
launched a drug with Schering-Plough Corp., Vytorin, that combines Zocor
and
Schering-Plough's Zetia to attack cholesterol levels in two complementary
ways.
The Vioxx recall stands to benefit Pfizer Inc., the world's biggest
drugmaker.
Merck and Pfizer have been battling for market share, with Pfizer's
Celebrex
arthritis drug dominating the market with about $5 billion in U.S. sales
alone last
year. Pfizer shares were up 35 cents to $30.53 in afternoon trading on the
NYSE.
Pfizer issued a statement Thursday citing the "outstanding long-term
safety profile"
of Celebrex and saying that in a recent FDA-sponsored study of 1.4 million
patients, those who received Celebrex demonstrated no increased risk of
cardiac
trouble.
Vioxx was labeled with a warning about heart risks in 2001 after Merck's
own
study in 2000 uncovered the increased risk of heart attack and other
complications.
The Food and Drug Administration has been monitoring problems reported to
it
since then.
"This is not a total surprise," said Dr. Steven Galson, acting director of
the FDA's
Center for Drug Evaluation and Research.
Dr. Steven Abramson, director of rheumatology at New York University
Hospital
for Joint Diseases, said "there are very few patients for whom there won't
be a
good alternative drug."
Besides generic anti-inflammatory drugs such as ibuprofen, naproxen and
aspirin,
those include Celebrex, which Abramson said has not been linked to heart
complications.
Celebrex and its successor drug, Bextra, as well as Vioxx and a successor
drug
called Arcoxia that is awaiting FDA approval, are part of a class of anti-
inflammatory drugs touted by the pharmaceutical industry as being more
effective
and having less side effects, particularly on the gastrointestinal system,
than older
drugs.
Vioxx's removal will be a blow to hopes that it and other drugs in the
class known
as COX-2 inhibitors could be used to prevent cancer in people at high risk
of
developing it. A landmark study in 2002 showed that small, daily doses of
aspirin
could prevent colon cancer, and studies hinted that COX-2 inhibitors might
do the
same possibly without aspirin's side effects.
All COX-2 inhibitors can raise blood pressure, but Vioxx appears to be the
only
one that's been linked to higher risk of heart attacks and strokes, Galson
said.
Merck is scheduled to release financial results for the third quarter,
which ends
today, on Oct. 21.
6.) A Case of Rofecoxib-Associated Stevens-Johnson Syndrome With
Corneal and Conjunctival Changes.
Goldberg D, Panigrahi D, Barazi M, Abelson M, Butrus S.
*Department of Ophthalmology, The Georgetown University-Washington Hospital
Center, Washington, DC; and dagger The Eye Research Institute, Harvard Medical
School, Boston, MA.
PURPOSE: To report a case of rofecoxib (Vioxx(R))-associated Stevens-Johnson
syndrome with corneal and conjunctival changes. DESIGN: Interventional case
report. METHODS: Case report of a 62-year-old woman with systemic lupus
erythematosus (SLE) taking rofecoxib for arthritis for 3 weeks. RESULTS:
Stevens-Johnson syndrome after 3 weeks of rofecoxib therapy. CONCLUSION:
This case report suggests that oral rofecoxib may trigger Stevens-Johnson
syndrome, potentially causing symblepharon, corneal neovascularization and
cicatricial ectropions.
7.) Pfizer Affirms Celebrex Safety ???????????
Source: Http://healthorbit.ca/
journal@lists.healthorbit.ca <journal@lists.healthorbit.ca>
NEW YORK, September 30, 2004 -- In response to Merck & Co.'s
announcement today of the worldwide withdrawal of its COX-2 medicine
Vioxx,
Pfizer Inc issued the following statement:
=======================================================================
DATA-MEDICOS/DERMAGIC-EXPRESS/SEPTEMBER JOURNAL 2.004/ DR. JOSE LAPENTA R.
=====================================================================
Produced by Dr. José Lapenta R. Dermatologist
Venezuela
1.998-2.024
Producido por Dr. José Lapenta R. Dermatólogo Venezuela 1.998-2.0024
Tlf: 0414-2976087 - 04127766810


















.png)